
Describe an experiment to verify Boyles’ law

Describe an experiment to verify Boyles’ law
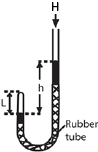
- air in the closed limb of a U-tube barometer as shown above
- mercury is poured to a height, h, and the length of the air column, L is noted.
- the length h is varied to obtain different sets of values of h and L
- Pressure of the gas is calculated from P = (H + h)ρg where H= height of barometer corresponding to atmospheric pressure, ρ = density of mercury, g = acceleration due to gravity. Note that h can be positive or negative.
- If A is the cross section area, V =AL
- Values of h, L, P, V and 1/V are tabulated
- A plot of P against 1/V gives a straight line through the origin which verifies Boyle’s law.
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES heat A-level
TAGS Dr. Bbosa Science

Really outstanding content
Thanks
I really like the content here
Explanations are well elaborated and not cringy as some textbooks I’ve tried to make use of