
Explain the variations of boiling points of chlorides of period period 3 elements

Boiling points of chlorides of period 3 elements
The table below shows the boiling points of the chlorides of period 3 elements.
| Element | Na | Mg | Al | Si | P | S | Cl |
| Atomic number | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Formula of the chloride | NaCl | MgCl2 | AlCl3 | SiCl4 | PCl3 | S2Cl2 | Cl2 |
| Boiling point (oC) | 1465 | 1418 | 180 | 57 | 76 | 136 | -35 |
A graph of boiling points of the chlorides against atomic number of the elements.
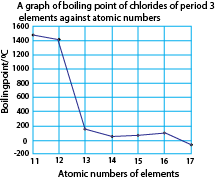
Explanation
NaCl and MgCl2 have high boiling points due to strong the ionic bonding.
Aluminium chloride, silicon chloride, phosphorus chloride, disulphur dichloride and chlorine have low boiling points because their molecules are held by weak molecular forces.
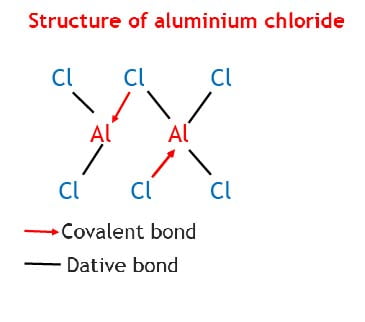
AlCl3 has a fairly high boiling point because in the liquid state, it consists of Al2Cl6 molecules and not simple AlCl3. These molecules are produced through dative bonding between Al and Cl in the Al2Cl6 molecules.
Disulphur dichloride has relatively high boiling point due to its high molecular mass that increases the strength of the molecular forces
Please Subscribe to promote this website. Subscription is free
Share with a friend
We value your comments
Thank you so much

Digital teachers have helped me to over hardship in A-level thanks for the effort you invest in provide a good service to students the almighty bless the admin abundantly
I will really be thankfull for your service sir
The best
I really appreciate your efforts