
formation of alkylhalides from alcohols

Formation of alkyl halides from alcohols
Alcohols react with a variety of reagents to yield alkyl halides. The most commonly used reagents are hydrogen halides (HCl, HBr and HI) phosphorus tribromide (PBr3) and thionyll chloride.
Examples
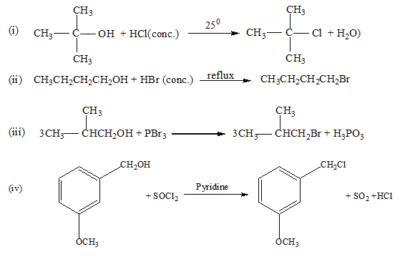
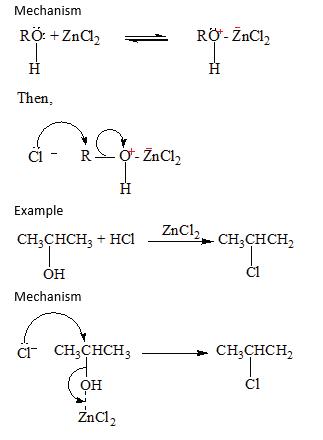
The order of reactivity of hydrogen halides is HI> HBr> HCl whereas the order of reactivity of alcohols is 30> 20> 10. The reaction of HCl with alcohol is catalyzed by anhydrous zinc chloride. The reaction is used to distinguish between primary, secondary and tertiary alcohols.
- Tertiary alcohol react readily with HCl in presence of anhydrous zinc chloride to form an insoluble chloride giving two layers immediately.
- Secondary alcohols form two layer in 50 -10minutes
- Primary alcohol do not form layers at room temperature.
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES Organic chemistry
TAGS Dr. Bbosa Science
