
Cell physiology (diffusion, osmosis, active transport, phagocytosis, pinocytosis) (A-level biology)

Cell Physiology
Overview
Materials move in and out of the cell by the following processes: osmosis, diffusion, active transport, phagocytosis, and pinocytosis. Some of these processes require energy while others do not. The materials include water, gases, enzymes, hormones, antibodies, and solutes
General objectives
By the end of the topic, the learner should be able to
- Explain the physiological process by which materials move in and out of cells
- Explain the role of these processes in the life of an organism
Specific objectives
The learner should be able to
- Describe osmosis, diffusion, active transport, phagocytosis and pinocytosis
- State the factors that affect the process of diffusion
- Describe the process of osmosis
- Explain the significance of diffusion and osmosis in organisms
- Explain how solvents and solutes are exchanged in animal and plant tissue or cell across the cell membrane in relation to its structure.
- Describe how unicellular organisms obtain water and food.
- Explain the relationship between structure and function of cell membranes
Practical
- Identify habitats with suitable media for the organism’s survival.
- Demonstrate the use of salt in food preservation.
- Demonstrate the use of visking tubing, glass column, and microscope in diffusion and osmosis experiments.
- Demonstrate conditions affecting the rate of diffusion
- Demonstrate the effects of osmosis on the cell or tissue
This refers to the processes by which materials get in and out of the cell. These processes include:-
- Diffusion
- Osmosis
- Phagocytosis
- Pinocytosis
- Active transport
Diffusion
It is the movement of particles (molecules or ions) from a region where they are comparatively concentrated to a region where they are at lower concentrations. The difference in the concentration between the two regions is called the concentration gradient or diffusion gradient. Diffusion will always take place whenever such a gradient exists, and it will continue until eventually the particles are uniformly distributed throughout the system.
It is a passive process that takes place by random thermal motion.
Functions of diffusion
- Gaseous exchange at the lungs
- Absorption of glucose and amino acids from intestine
- Absorption of water from the colon
- Uptake of glucose by cells from blood: Glucose does not diffuse freely through the cell membrane because it is insoluble in lipids. It passes through the cell membrane is facilitated by proteins. Therefore, diffusion of glucose through the cell membrane is called facilitated diffusion.
- Gaseous exchange for photosynthesis
- Loss of water during transpiration
- Diffusion of flower scent to attract insect pollinators.
- Absorption of ions from the soil
Factors that affect the rate of diffusion
- Distance over which diffusion occurs: the bigger the distance the lower the rate of diffusion
- Temperature: the higher the temperature the faster the rate of diffusion because particles have high kinetic energy.
- The extent of the concentration gradient: The greater the difference in concentration, the more rapid the diffusion. The closer the distribution of the material gets to equilibrium, the slower the rate of diffusion becomes.
- Mass of the molecules diffusing: Heavier molecules move more slowly; therefore, they diffuse more slowly. The reverse is true for lighter molecules.
- Solvent density: As the density of a solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium. If the medium is less dense, diffusion increases. Because cells primarily use diffusion to move materials within the cytoplasm, any increase in the cytoplasm’s density will inhibit the movement of the materials. An example of this is a person experiencing dehydration. As the body’s cells lose water, the rate of diffusion decreases in the cytoplasm, and the cells’ functions deteriorate. Neurons tend to be very sensitive to this effect. Dehydration frequently leads to unconsciousness and possibly coma because of the decrease in diffusion rate within the cells.
- Solubility: As discussed earlier, nonpolar or lipid-soluble materials pass through plasma membranes more easily than polar materials, allowing a faster rate of diffusion.
- Surface area and thickness of the plasma membrane: Increased surface area increases the rate of diffusion, whereas a thicker membrane reduces it.
Osmosis
This is the passage of solvent molecules from a region of their high concentration to a region of their low concentration through a partially permeable membrane. The solvent in a biological system is water.
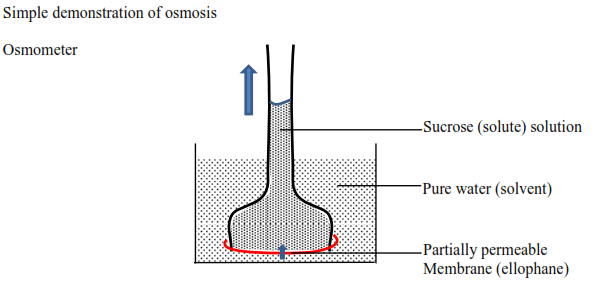
The solid arrows indicate the net flow of water (solvent) into the solution. The membrane is partially permeable, allows water molecules to pass into the thistle funnel from the beaker. As a result of the net flow of water into the funnel, the solution rises up the tube as indicated by the arrow.
Water potential, Ψ (Psi)
This is the capacity of the system to lose water. At standard temperature and pressure pure water is given a water potential of Zero.
Adding a solute to water lowers the water potential, making it negative. This is because, the presence of solute molecules lowers the concentration of the water molecules, thus, reducing the number of water molecules that can diffuse out of it. Further adding of a solute lowers the water potential, making it more negative.
When this happens, water moves to equilibrate, moving from the system or compartment with a higher water potential to the system or compartment with a lower water potential.
Osmotic pressure
This is the pressure that is required to prevent the net movement of pure water into an aqueous solution through the differentially permeable membrane. The osmotic pressure increases as the concentration of the solute increases. In other words, osmotic pressure is inversely proportional to water potential.
Osmosis and cells
Cell membranes are differentially permeable.
- If a cell is surrounded by pure water or a solution whose solute concentration is lower than that of cell content, the water molecules will pass into the cell by osmosis. The volume of the cell increases and the cell swells up; in this case, the water potential of the external solution is higher and the osmotic potential lower than that inside the cell. The external solution is said to be hypotonic.
- If the cell is surrounded by a solution whose solute concentration exceeds that of the cell, water passes out of the cell and the volume reduces and consequently shrinks. The external solution is hypertonic.
- If the solute concentration of the solution and the concentration of the cell constituent are equal, osmosis does not occur, the solution is isotonic.
Effects of osmosis on animal cell e.g. Red blood cell
A solution of 0.9% sodium chloride is isotonic with human cells, and if red blood cells are immersed in isotonic solution, they will neither swell nor shrink. When placed in a hypotonic solution, red blood cells swell and burst or hemolyze or undergo hemolysis.
On the other hand, a red blood cell immersed in a hypertonic solution will shrink and plasma membrane crinkles. This is known as crenation.
It follows that if a cell is to maintain its normal size and shape, it must exist permanently in an isotonic solution or failing that, it must have special mechanism enabling it to survive in a hypertonic or isotonic solution.
This special mechanism is called osmoregulation. For example, freshwater Amoeba would undoubtedly swell up and burst, just like a red blood cell in water were it not for a contractile vacuole that expels excess water from the body.
Effects of osmosis on plant cell
When the plant cell is surrounded by a hypotonic solution, water enters the vacuole by osmosis, the cell swells as the volume increases. However, it does not burst. This is because the cellulose cell wall stretches and develops tension, resisting the further expansion of the cell.
As water flows into the vacuole by osmosis, the tension developed by the cell wall causes an internal hydrostatic pressure to develop. This is called pressure potential and it opposes the continued uptake of water into the cell by osmosis. The pressure potential reaches a maximum when the cell wall is stretched as much as possible and can stretch no more. At this point the cell is said to be fully turgid or maximum turgor is reached.
Turgidity in the cell provides support and maintains the shape and form of plant parts.
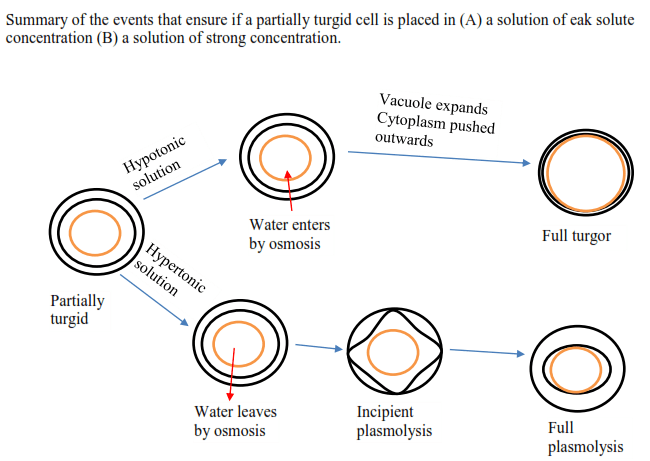
When plant cells are immersed in a hypertonic solution, water is drawn out of the cell. This leads to a decrease in the volume of the cell. In a few minutes, the protoplast shrinks to such an extent that it pulls away from the cell wall leaving a gap between the cell wall and plasma membrane.
The shrinkage of protoplast from the cell wall is called plasmolysis. Plasmolysis sometimes happens to the plants exposed to extreme salty water but otherwise, it rarely occurs in nature.
The water relations of plant cell
In considering the water relation of a plant cell we need to take into account the following three pressures.
- The net water potential of the whole cell or water potential.
- The water potential of the solution in the vacuole or solute potential.
- The hydrostatic pressure caused by the cell wall pressing inwards against the cytoplasm or pressure potential
When a plasmolyzed plant cell is transferred in pure water, the water will immediately enter the cell sap in the vacuole by osmosis. The protoplasm will begin to expand against the cytoplasm, so the pressure potential is zero and the water potential equals the osmotic potential of sap. As the influx of water continues, the protoplast goes on expanding until the cell membrane comes in contact with the cellulose wall. When this occurs, the influx of water starts to be opposed by the inward pressure of the cell wall, i.e. the pressure potential. The water potential of the cell now becomes less negative than the osmotic potential of the cell sap by the amount of pressure potential. As the cell continues to expand, the pressure potential gets steadily greater and water potential of the cell gets less and less negative. Eventually, full turgor is reached. The cell can expand no more, the water potential of the cell reaches zero and the water potential of the sap is exactly counterbalanced by the pressure potential.
A graph illustrating the relationship between the water potential of the cell (Ψcell), Osmotic potential of the cell sap (Ψs) and pressure potential (Ψp) of a plant cell at different states of turgor and plasmolysis.
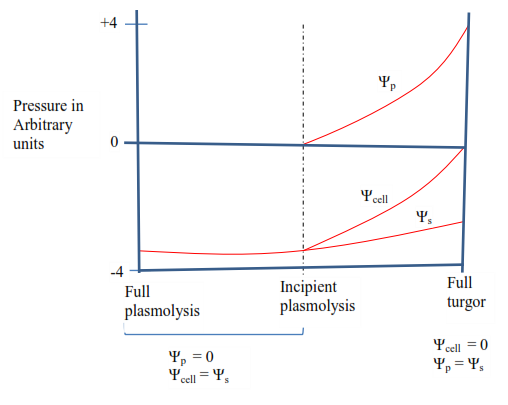
The water relationship of a plant cell can be summarized by the following equation

Measuring water potential of cells
- cut living tissue of equal mass or volume and measure them.
- Place the tissues in a series of solutions of different solute concentration
- Allow the tissues to stay in the solution for a certain period of time
- Remove the tissues from the solution and measure the second time
The solution which produces no change is the solution with the same water potential as the tissue. Therefore, the osmotic potential is equal to the water potential
When a plant cell is placed in a hypotonic solution, water enters its vacuole by osmosis. This makes the volume of the protoplast to increase, so causing it to press against the cell wall. This outward pressure, called turgor pressure, stretches the cell wall and opposed by an inward pressure (pressure potential) exerted by the cell wall against the cell’s contents.
Turgor pressure reaches its maximum valves called full turgor pressure when the cell wall cannot be stretched anymore. At full turgidity, the pressure potential (Ѱp) is equal and opposite to the osmotic potential (Ѱs) of the cell, i.e. Ѱp = Ѱs ( since Ѱs is always negative)
Note: when a plant cell placed in a hypertonic solution, water leaves the cell vacuole by osmosis. As a result, the protoplast shrinks and eventually pulls away from the cell wall, a process called plasmolysis. The point at which plasmolysis is just about to happen, i.e. when the protoplast (cell membrane) is just about to lose contact with the cell wall is referred to as incipient plasmolysis
Wilting
When the cell in the stem and leaves of a plant lose more water by evaporation than they can absorb, turgor is reduced and the plant visibly droops. This phenomenon is called wilting.it can often be observed on hot, dry and windy days. The plant recovers at night as evaporation is reduced and stomata closed; but if the water supply to the root is inadequate, the plant dies.
Functions of osmosis
- Absorption of water by the root hair
- Absorption of water in the loop of Henle
Active transport
It energy-consuming transport of molecules or ions across a membrane against a concentration gradient. Substances usually transported across cell membrane by active transport include Na+, K+, ureate ion and amino acids
The following observation suggests evidence of the use of energy in active transport.
- It is only found in living system which is continuously producing energy by respiration.
- Increase in temperature and oxygen concentration increases the rate of active transport
- When formation or use of ATP is inhibited by such agents as cyanide, active transport will not take place
- Cells that take part in active transport contain a large number of mitochondria.
Functions of active transport
- Absorption of minerals in the stomach
- Absorption of minerals in ascending loop of Henle
- Absorption of mineral in the root hair from the soil
- Entry of water into the guard cell leads to their opening
Endocytosis and exocytosis
These are process by which larger objects are taken into or expelled from the cells
Exocytosis
This provides a means by which enzymes, hormones, antibodies, and cell wall precursors are released from the cells. Here a vesicle containing the material moves towards the surface of the cell and fuses with the plasma membrane. The vesicle opens to the exterior and its contents leave the cells
Endocytosis
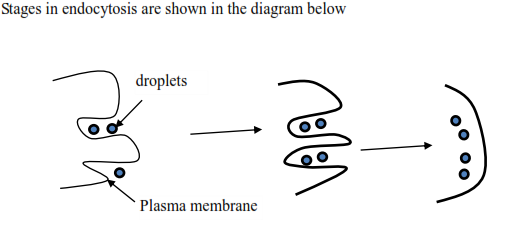
This provides a means by which big objects are taken by the cell. First, the plasma membrane invaginates to form a flask-shaped depression that envelops the material. The neck of the flask then closes, and the invagination becomes sealed off to form a vesicle that moves into the cell. When a liquid-like substance is taken in by the cell the process is referred to as pinocytosis. And solid particles are taken in by phagocytosis.
For revision questions and answers download PDF

It’s hard to find knowledgeable individuals on this topic, but you sound like you understand what you’re talking about! Thanks