
Period II, diagonal relationship (A-level inorganic chemistry)

Diagonal relationship
This is the similarity in chemical properties between elements in the second period with elements which are lying to their right in the 3rd period or lying diagonally opposite to them.
The diagonal relationship
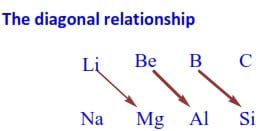
Li and Mg, Be and Al, B and Si have similarities in their chemical properties.
Such similarities in chemical properties between the first element and the second element in the next higher group are called diagonal relationship. This kind of relationship exists between some elements of periods 2 and 3.
Reasons for diagonal relationship
The two diagonally related elements (e.g., Li and Mg or Be and Al) have very similar electropositivity.
Electropositivity increases down the group and decreases across a particular period, the increase in electropositivity in going down one place in the periodic group, (e.g., magnesium is one place lower than lithium) is compensated for by the decrease that occurs in moving one step across a period from left to right (magnesium is one place to the right of lithium).
Alternatively, two diagonally related elements have the same polarizing power since on moving from left to right across a period, the cationic charge increases and cationic size decreases, the magnitude of polarizing power increases, and moving down the group, however, cationic size increases reducing the polarizing power. Since these two variations of polarizing power of cations (along the period and down the group) are opposite each other, they partially cancel (balance) each other when we move diagonally in the periodic table.
Alternatively, two diagonally related elements have the same electronegativity, since an increase in electronegativity one step across the period is cancelled out by a decrease in electronegativity one step down the group.
Similarities between Li and Mg
- Both form normal oxides when they burn in oxygen.
4Li (s) + O2 (g) → 2Li2O (s)
2Mg (s) + O2 (g) → 2MgO (s)
2. They combine with nitrogen to form nitrides.
3Mg (s) + N2 (g) → (Mg2+)3(N3-)2 (s)
6Li(s) + N2 (g) → 2Li3N(s)
3. They combine with carbon to form carbide
Mg (s) + 2C (s) → Mg2+(CºC)2- (s)
2Li(s) + 2C(s) → Li2C2(s)
Others
4. Their carbonates, hydroxides and peroxides readily decompose to the oxides on heating.
Li2CO3 (s) → Li2O (s) + CO2 (g)
MgCO3 (s) → MgO (s) + CO2 (g)
5. Their nitrates decompose on heating to give oxides, nitrogen dioxide and oxygen.
4LiNO3 (s) → 2Li2O(s) + 4NO2 (g) + O2 (g)
2Mg(NO3)2 (s) → 2MgO(s) + 4NO2 (g) + O2 (g)
6. Their carbonates, oxalates and phosphates are sparingly soluble in water.
7. Form strongly hydrated ions in solution
8. Chlorides are deliquescent Chlorides are soluble in ethanol
Trial 1
(i) Explain what is meant by diagonal relationship. (3 ½ mark)
(ii) Name five properties in which lithium resembles magnesium but differs from the rest of group 1 metals. Give one reason for the resemblance between lithium and magnesium. (10 marks)
Similarities between Be and Al.
- Both are passive to nitric acid.
- Both react with NaOH solution to evolve hydrogen.
Be (s) + 2–OH (aq) + 2H2O (l) → Be(OH)42-(aq) + H2 (g)
Al (s) + 2–OH (aq) + 6H2O (l) → 2Al(OH)4– (aq) + 3H2(g)
Others
3. The oxides and hydroxides of beryllium and aluminium are amphoteric.
4. The chlorides are covalent polymeric solids (through dative bonding). When anhydrous (BeCl2)X and (AlCl3)X which readily dissolve in organic solvents. They are readily hydrolyzed by water, with evolution of HCl.
5. Beryllium carbide, Be2C, and aluminium carbide, Al4C3, give methane on treatment with water.
Be2C(s) + 4H2O(l) → 2Be(OH)2(s) + CH4(g)
2Al4C3(s) + 12H2O(l) → 4Al(OH)3(s) + 3CH4(g)
6. Similar complexes of beryllium and aluminium have similar stabilities, e.g., BeF42- and AlF63-.
Trial 2
Beryllium differs in some of its properties from the rest of the elements in the group.
(i) State two properties in which beryllium differs from the rest of the members of the group. (2marks)
(ii) Give reasons why beryllium shows different properties from the rest of the elements. (2marks)
Trial 3
Explain the following observations.
(b) Beryllium belongs to group II of the periodic table and yet its Chemistry and that of its compounds resemble that of aluminum.
Trial 4
Beryllium, like aluminium can react with sodium hydroxide solution. Other group II elements do not.
(i) Write ionic equations for the reactions of beryllium and aluminium with sodium hydroxide solution.
(ii) List three other properties in which beryllium shows similarity to aluminium.
(iii) Explain why beryllium behaves differently from other group II elements.
(iv) Name two other elements which have a similar relationship like beryllium and aluminium.
Similarities between B and Si
- Non-metals with very similar properties; non-conducting.
2. Compounds are covalent.
Their oxides (Be2O3 and SiO2) are strongly acidic in character and form oxy-acids (H3BO3, H2SiO3) with water. These react with aqueous alkalis to produce borates and silicates.
Form covalent chlorides, BCl3 and SiCl4, which are readily hydrolyzed Form borides, e.g., MgB2 and CaB6, and silicides, e.g., Mg2Si, with metal.

I’m having a little problem I cant subscribe your rss feed, I’m using google reader fyi.
you can suscribe via our website: digitalteachers.co.ug
The notes are good
Please can I have the PDF?
check on the website “digitalteachers.co.ug”
You are my model thanks