
Alkyne by Dr. Bbosa Science (A-level inorganic chemistry)

Alkyne
General formula CnH2n-2 where n≥2
They contain a triple bond
Examples


Preparations of alkynes
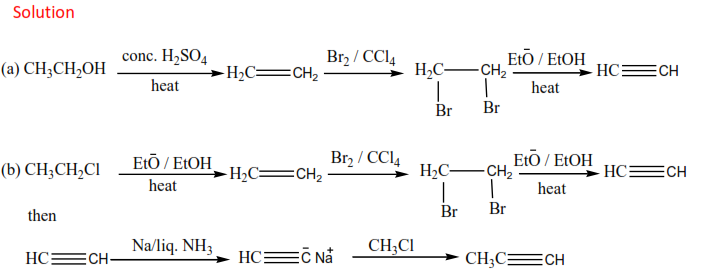
1. From vicinal dihalides.
Vicinal dihalides are halides with two halogen atoms on adjacent carbon atoms
The reaction is carried out by refluxing vicinal dihalides with a mixture of an alcohol with its sodium or potassium hydroxide.
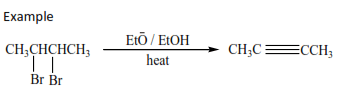
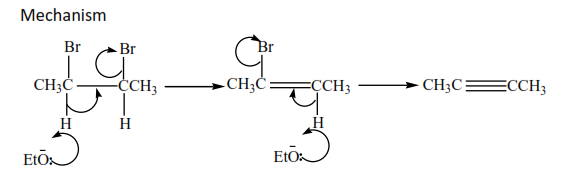
2. Preparation of alkyne from carbon
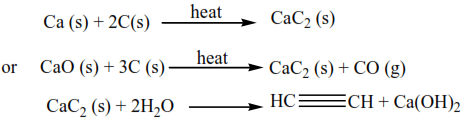
3. Preparation of long-chain alkyne from ethyne

Exercise
Synthesize
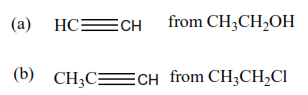
Questions involving synthesis requires a student to write a series of reactions leading the formation of products from the reactant(s). In most cases, more than one equation is required because reactions of organic compounds are specific that we may need to convert the reactants into intermediate compounds before a product can be obtained.
Physical properties
1. They range from gases to liquids to solids
2. They are insoluble in water but soluble in organic solvents
Chemical properties.
1. Alkyne burn in oxygen to give carbon dioxide, water, and heat.
Example
2C2H4 + 5O2 → 4CO2 + 2H2O + heat
They are used as fuel because they produce heat
2. Reduction of alkyne
Depending on conditions, alkyne may be reduced to alkenes or alkenes

Note that the reduction of alkyne to alkene has synthetic value because alkenes are more reactive than alkanes.
Example
Synthesize
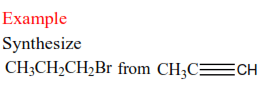
Solution
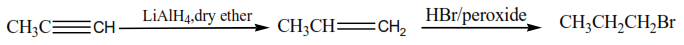
Not that the first part of the synthesis involves reduction alkynes to alkene.
3. Addition reactions
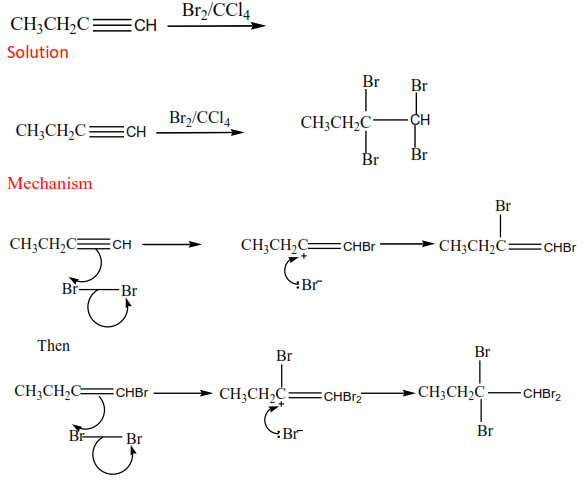
Alkynes undergo addition reactions like alkenes except that the addition occurs twice. (a) Addition of HX (X= Cl, Br, and I
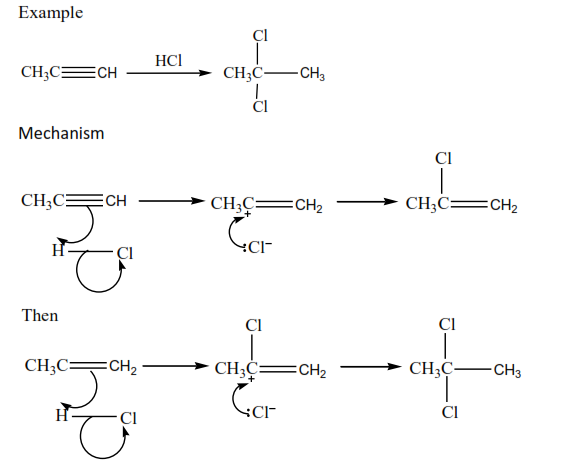
The halogen is diluted with carbon tetrachloromethane (to prevent explosion) Example
Exercise
Complete and write a mechanism
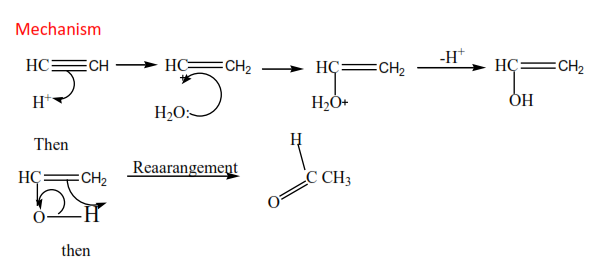
(c) The reaction of alkyne with of water.
The reaction is catalyzed by mercury (II) sulphate and dilute sulphuric acid. Carbonyl compounds are formed.
Example

Mechanism
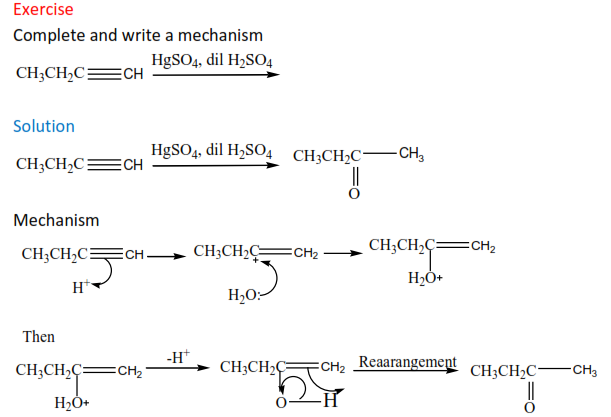
(d) The reaction of a terminal alkyne
Alkynes with a triple bond at the end of the chain react with ammoniacal silver nitrate or ammoniacal copper I chloride to form white precipitate or red precipitate respectively.
Example
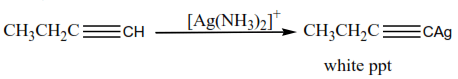
This reaction is used to distinguish alkynes with a triple bond at the end of those with a triple bond in the middle of the chain.
Exercise
Name one reagent that can be used to distinguish between the following pairs compounds. In each case state what will be observed when the reagent is treated with each of the compounds of the pair.
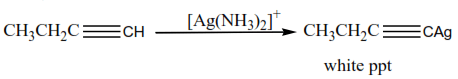
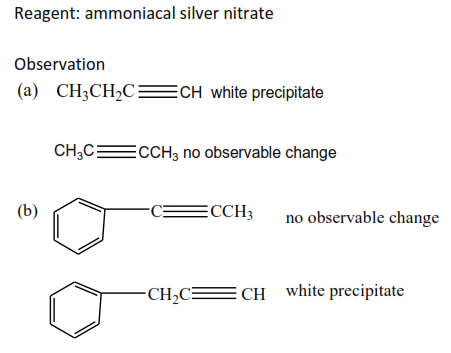
Revision questions
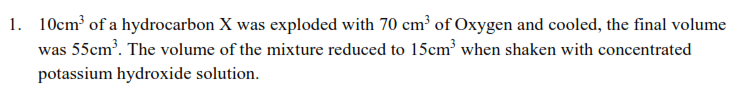
.
(a) Determine the molecular formula of X. (b) Write and name all isomers of X.
(b) X formed a red precipitate with ammoniacal copper 1 chloride, (i) Identify X.
(ii) Write equations and suggest mechanism for the reaction between X and acidified
water, hydrogen in presence of palladium catalyst and hydrogen peroxide. (d) Write equations and conditions to show how X can be prepared from a named
(i) Alcohol
(ii) Alkyl halide
