
Properties of matter – upper primary science

PROPERTIES OF MATTER
Matter
Matter is anything that occupies space and has mass. Mass is what matter is made up of. Matter exists in three states commonly known as solids, liquids and gases.
Characteristics of matter
The different states of matter have different characteristics in regard to shape, volume and mass.
- Solids have a definite shape, volume, and mass. Examples of solids are brick, sand, shoe
- Liquids unlike solids have no definite They take the shape of the container they occupy. However, have definite volume and mass. Example of liquids are water, milk, petrol.
- Gases like liquids have no definite They take the shape of the container they occupy. They have no definite volume and compressed. They however have mass.
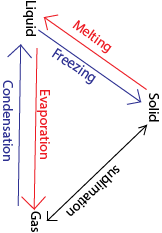
Effects of heat on matter
Effects of heat on solids
When solids are heated the following things may take place:
- expands or increase in size
- melts or change into liquid
- sublime or changes directly to gas without going through a liquid
- on cooling the solid contraction .
Effects of heat on liquids
When the liquid is heated it evaporates into a gas. On cooling the gas condenses into a liquid or sublimes into a solid.
Summary of effect of heat no matter
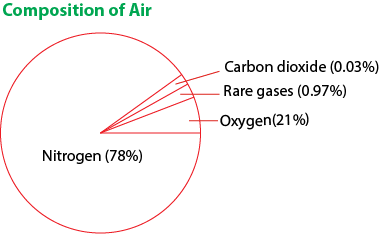
Air
Air is a mixture of gases i.e.
- nitrogen 78%
- oxygen 21%
- inert gases 0.97%
- carbon dioxide 0.03%
- Note that air contains certain varying amounts of water and dust particles.
| Component of air | Used for |
| Oxygen | Breathing, burning, rusting and germination |
| Carbon dioxide
|
Photosynthesis (Making plant food), preserving soft drinks, putting out fire (fire extinguishers), |
| Nitrogen | Used by legumes to fix nitrogen into the soil |
| Inert gases | Used in electric bulbs or tube |
Wind
This is moving air
Effects of moving air (wind)
Wind as negative effects on our surroundings particularly when it is very strong. Some effects of winds are:
- blowing soil away (erosion)
- blowing roofs away
- felling trees
Uses of moving air (wind)
We normally make use of wind in many ways. Some uses of wind are:
- Winnowing – this is the process of separating chaff from grains by using the The grains are tossed in the air and if the wind is blowing the chaff are blown away by the wind leaving the grains. One can also blow
away the chaff using the mouth. - Sailing boats and canoes –some boats and canoes have sail (a large piece of very strong cloth) that they use to catch the wind in order for the boat or canoe to move forward,
- Turning windmills – a windmill is a structure that has blades attached to its roof and is driven by the wind
in order to turn and drive a machine attached to it. A wind mill can be used to generate electricity.
Experiment to show that air occupies space
- When air is blown into a balloon, the balloon expands.
- When the glass is inverted in a basin of water, the water does not enter the glass because air occupies space in glass

3. Air bubbles out of an empty jerry can or bottle before water enters.

Experiment to show that air has weight.
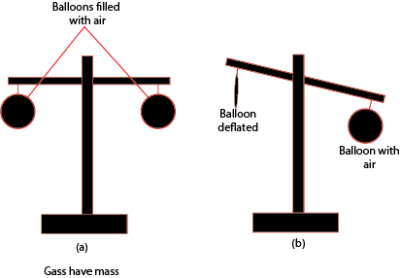
A deflated balloon is lighter than a balloon that contain air
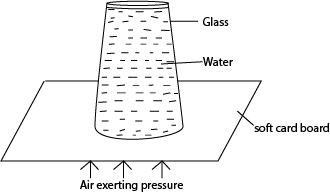
Experiment to show that air exerts pressures
Density of substance
Density is mass / volume
Mass is object is determined by weighing balance

Volumes of regular objects can be determined from the formulas
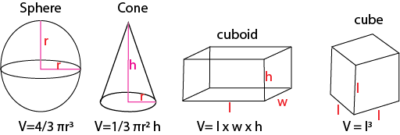
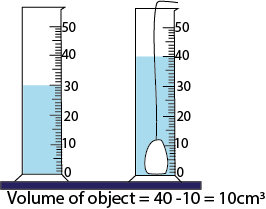
Volumes of irregular objects
- Volume using displacement method
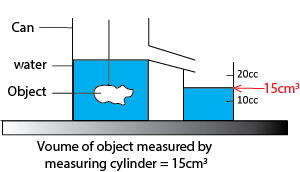
- Using overflow can
Example
Find the density of acube of side of 2cm3 and mass of 10g
Volume = l x w x h = 2 x 2 x 2 = 8cm3
Density = mass/volume = 10/8 =1.25gcm-3
Note that the density of water = 1gcm-3 or 1000kg/m3
Floating and sinking in a liquid
A substance denser than a liquid sinks while that less dense than a liquid float,
A substance that sinks displaces amount of a liquid equal to its volume.
A substance that float displace amount of a liquid equal to its weight.
For example stones, metals sink in water because it is denser than water while paraffin floats in water because it is lighter than water.

Pressure in liquids
The force that makes the liquid to flow is called pressure. The refusal of the difficult of a liquid to flow is viscosity. Pressure in liquids increase with depth as shown in the diagram below
Pressure increases with depth
The diagram shows that the jet at the bottom of the container (3) has more pressure than all the others. This is because it is the deepest among the others. Therefore the pressure increases with depth.

Mixtures
A mixture is a combination of two are more mixture
Examples
- Solid mixture such as maize and bean
- Suspension is made of insoluble solid powder in a liquid
- Solution is made of a two miscible liquids such a petro and paraffin or a soluble solid in a liquid such as sugar in water. The liquid that dissolves a solid is called a solvent while a solid that dissolves in a liquid is a solute
Methods of separating mixtures
- Winnowing – the wind is used to separate chaff, small sticks, and dry leaves, from grains or seeds by blowing the chaff away. Chaff are usually lighter than the grains.
- Sieving – a sieve is used to separate mixtures of particles of different For example stones from flour. The larger particles remain on the sieve while the finer ones pass through.
- Picking /sorting– Here the large particles are separated from smaller ones by observing them and using the hands to remove the unwanted ones. For example stones are separated from rice or beans in this way.
- Filtering- this method is used to separate a liquid from particles that cannot dissolve in it. For example separating sand from water using filter paper or piece of cloth.
- Decanting – this is when a liquid is separated from large particles of solids by pouring the liquid out For example pouring off kerosene that has mixed with tiny ball bearings.
- Using a magnet – a magnet is used to separate magnetic materials that may have mixed up with powder or other non-magnetic materials. For example a magnet is used to separate iron fillings from sugar.
- Evaporation- a solid that has dissolved in a liquid is separated in this The liquid is heated until it evaporates and the solid is left behind, For example salt dissolved in water can be recovered by evaporation.
- Miscible liquids are separated by a separating funnel
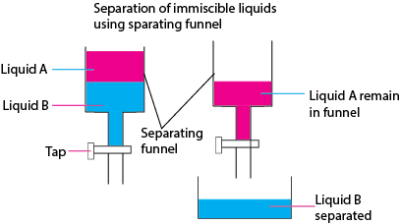
Physical and chemical changes
Physical and chemical changes
A physical change: Is one in which no new substance is formed.
Examples of physical change are
- Melting of wax
- Melting of ice
- Boling of water
- Expansion of metal
- Evaporation
- Sublimation of iodine, ammonium chloride, iron (III) chloride
A chemical change:
Is one in which a new substance is formed.
In many chemical changes, heat and light are given out.
In a chemical change, it is difficult or impossible to change the new substance back to their original composition.
Examples of chemical changes
- Burning of candle wax
It requires oxygen to take place
- Rusting
Rusting
When iron is left in damp air for some time it become covered with a brown coat called rust. Chemically rust is hydrated iron III oxide Fe2O3.xH2O.
Disadvantage of rusting
- Weaken objects made of iron
- Makes objects made of iron look ugly
Conditions necessary for rusting
- Oxygen not air
- Water
Experiment to show that oxygen and water is necessary for rusting

The experiment is set up as shown above and left for several days
- Test tube A contains nails and moist air
- Test tube B contains nails and dry air because moisture is removed by anhydrous calcium chloride
- Test tube C contains nail and air free water; boiling removes dissolved air from water while a layer of oil prevents entry of air into water
Observation after several days
- In test tube a rusting took place because there is both oxygen and water necessary for rusting to take place.
- In test tube B rusting did not take place because there was not water
- In test tube C rusting did not take place due to absence of air
Conclusion
Both oxygen and water are necessary for rusting to take place
Method of preventing rusting
- Keeping iron and steel equipment in air or water free environment, i.e., in the dry places (from water).
- Oiling (protects equipment from water and oxygen).
- Painting (protects from both air and water)
- Tin plating protects iron from both air and water; however, tin-plate is only effective provided the layer of tin remains intact
- Galvanizing: this is coating iron with zinc. Zinc protects iron because it is passive in air but also it can reduce iron III to ion.
- Alloying
An alloy is a mixture of two or more metals
Examples of alloys
| Alloy | Composition | uses |
| Steel | Iron and carbon | Making cooking utensils, bridges |
| Bronze | Copper and tin | Ornaments, coins |
| Brass | Copper and zinc | Coins, ornament |
| Solder | Tin and lead | Welding |
For revision questions and answers download PDF below
PROPERTIES OF MATTER – upper primary
Sponsored by The Science Foundation College +256 753 80 2709
Compiled by Dr. Bbosa Science +256 778 633 682

Thanks so much digital teachers for the great work done ✅
You always make things easy to understand. 500 ka redeem code
Take the first step toward success with MBBS Direct Admission in Tamil Nadu.