
Separation of mixtures (O-level chemistry)

Separation of mixtures
A mixture is a substance, which consists of two or more substances/elements or compounds NOT chemically combined together.
Examples of mixtures include;- Ink, milk, paint is common examples of the mixture. Note that all solutions are mixtures.
A compound is a substance, which consists of two or more elements chemically combined together.
Example
– Water is a compound of the elements hydrogen (H) and Oxygen (O)
– Common salt (sodium chloride) is a compound of the elements sodium (Na)
and Chlorine (Cl)
An element is a substance, which cannot be split/divided up into simpler substances by chemical means.
Common elements are: – oxygen, nitrogen, sulphur, carbon, iron, copper
Methods of separation of mixtures
1. Crystallization:
This is the process of obtaining pure crystals from the solution.
Definition
A solution is a uniform mixture of two or more substances.
A solute is the dissolved substance, e.g., sodium chloride.
A solvent is a substance that dissolves a solute, e.g., ethanol, water, chloroform, etc.
Substances that dissolve in any solvent are said to be soluble in that particular solvent and those, which do not, are said to be insoluble substances and may settle down as sediments or may float in the solvent. E.g., chalk. They form what we call a suspension.
A suspension is a liquid containing small particles of solid spread throughout it and which settle on standing.
A saturated solution of a solute at a particular temperature is one which can dissolve no more solute in the presence of the solute.
A suspension differs from a solution in three ways:
- It contains solid particles that can be seen.
- Its solid particles settle on standing and
- Filtration separates it into the filtrate and a residue.
2. Sublimation
It is a process whereby a solid, on heating changes directly into the vapor state without first becoming liquid and on cooling condenses directly into the solid form without passing through a liquid state.
Substances like iodine, iron (III) chloride, Ammonium chloride that sublime can be separated from those that do not sublime by heating the mixture in a dry test tube. Iodine, iron (III) chloride or ammonium chloride would sublime leaving the nonvolatile substance as a residue in the test tube
Sublimation may be demonstrated by heating either iodine or ammonium chloride crystals in a dry test tube
Experiment: Action of heat on substances that sublime;
1). Iodine (I2)
Apparatus: Test tube, test tube holder
Materials: Iodine
Method: place iodine in a dry test tube and heat strongly
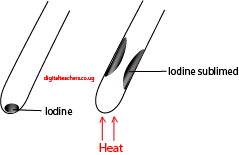
Observation: dense purple fumes of iodine sublime on the cooler parts of the test tube.
2). Ammonium chloride (NH4Cl)
Apparatus: Test tube, test tube holder
Materials: ammonium chloride
Method: place ammonium chloride in a dry test tube and heat strongly
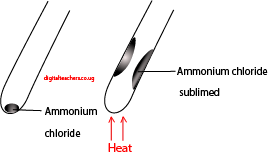
Observation: Dense white fumes of ammonium chloride sublime on the cooler parts of the test tube.
Experiment
Separation of ammonium chloride from sodium chloride
You are provided with a mixture of sodium chloride and Ammonium chloride. Place the mixture in a dry test tube and heat the mixture strongly.
(a) State your observation and conclusion
Experiment: Separation of ammonia chloride from sodium chloride
Apparatus
Test tube
Tongs
Material
A mixture of ammonium chloride and sodium chloride
Heat source
Method
Place the mixture of ammonium chloride and sodium chloride into a test tube and heat strongly.
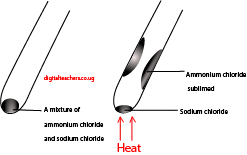
Observations:
Dense white fumes of ammonium chloride sublimed to the cool part of the test tube leaving sodium chloride at the bottom of the test tube.
Conclusion:
Ammonium chloride can be separated from sodium chloride by sublimation.
3. Magnetism
A magnet can attract materials (metals) because the metals/materials being attracted have got some magnetic properties. Magnetic substances may be separated from the non-magnetic substances by the use of a magnet.
EXPERIMENT: Separating of a mixture of iron filing from sulphur
You are provided with a mixture of iron and sulphur. Place the mixture on a plain sheet of paper. Use the bar magnet provided to separate the two. (State your method, observations, and conclusion)
Materials
Iron filings
Sulphur
Magnet
Method
- Place a mixture of sulphur and iron on sheet of paper
- Pass a magnet over the mixture.
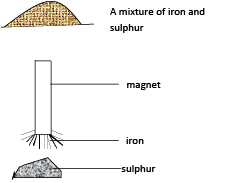
Observations
Iron filings are attracted to the magnet leaving sulphur on the sheet of paper.
Conclusion
Iron filings are separated from sulphur using a magnet because they have magnetic properties while sulphur does not.
4. Chromatography:
Is a process of separating colored substances by using porous material e.g. filter paper, based on their different rates of movement, using a suitable solvent e.g. alcohol (ethanol,), water.
– Colored materials found in leaves e.g. chlorophyll can be separated.
– The bands/lines/shades obtained after chromatography are referred to as chromatograms.
– The component which is more soluble in that solvent moves far away from the mixture.
Procedure
- A drop of a mixture of colored material such as ink or chlorophyll is placed in the middle of a filter paper
- Add a suitable solvent such as ethanol is dropwise. In each case, a drop is allowed to dry before another is added.
Observed a chromatogram of a ring of different colors is obtained because each colored substances have different solubilities and move at different rates.
Observation
The following colors were observed
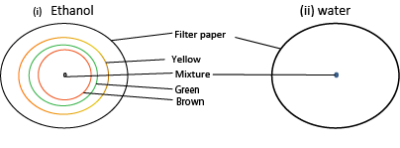
Chlorophyll dyes did not separate when water was used because water is not a suitable solvent.
Conclusion
Chlorophyll/ ink is not a single component and the components in chlorophyll/ ink can be separated by chromatography.
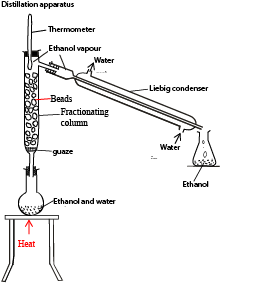
5. Distillation
Is a process of boiling a liquid to form a vapor, where the vapor condenses and collects as a distillate. The substance that forms vapor easily is said to be volatile.
Distillation is used to separate miscible substances (such as water and ethanol) that differ in the boiling points. A substance that has a lower boiling point turns into vapor easily and collected as a distillate.
6. Immiscible liquids
Immiscible liquids are those that do not form a uniform mixture; i.e. they form a separate layers. The less dense liquid settles on top.
Immiscible liquids are separated by a separating funnel

Separating funnel
Alloys:
Pure metals are not usually strong. Their appearance, strength and resistance to corrosion can be improved by mixing with other elements. The mixtures are called alloys.
An alloy is a uniform mixture of metal with one or more metals or carbon.
Examples of alloys
| Alloy | Composition |
| Steel | Iron (Fe) and carbon (C) |
| Brass | Copper (Cu) and zinc (Zn) |
| Solder | Lead (Pb) and tin (Sn) |
| Duraluminium | Magnesium (Mg), copper(Cu) and aluminium (Al) |
| Bronze | Copper (Cu) and tin (Sn) |
| Cast iron | Iron (Fe), phosphorus (P), sulphur(S), silicon (Si), manganese (Mg) |
| Wrought iron | Iron (Fe) and carbon (C) |
Experiment: To study the difference between a mixture of iron (Fe) and sulphur (S) and a compound of iron sulphide (FeS)
| Test | Observation | |
| Heat a mixture of iron and sulphur in a dry test tube. | The black solid formed. Fe (s) + S(s) → FeS (s) | |
| Appearance | Look at the mixture through a magnifying glass | – The elements can be seen separately in the mixture. – The compound is homogeneous. |
| Water | -Add a little of the mixture to water in a test tube, shake and allow to stand. – Repeat with the compound | – The metal in the mixture separates first because it is denser and the sulphur floats. – The compound does not separate completely. |
| Dilute acids | -Add dilute hydrochloric acid or sulphuric acid to the mixture in the test tube and warm gently. – Repeat with the compound. | -The acid reacts with iron (or zinc) in the mixture, forming hydrogen which burns with a’pop’ sound, sulphur does not react. Fe (s) + H2SO4 (aq) → FeSO4(aq)+ H2(g) The compound reacts to form hydrogen sulphide, which smells like bad eggs and burns quietly with a blue flame. FeS(s) + H2SO4(aq) → H2S (g) + FeSO4(aq) 2H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g) |
Differences between a mixture and a compound a mixture and a compound
| A mixture | A compound |
| – Energy is not given out or absorbed when a mixture is made. | Energy is given out or absorbed |
| – The substances in it can be separated by physical means. | The elements in it cannot be separated by physical means. |
| -Its composition is variable; the substances can be presented in any proportions by mass | Its composition is not variable; the elements are combined in definite proportions by mass. |
| -Its properties (e.g. color, density) are the average of those of the substance in it. | Its properties are quite different from those of the elements in it. |
For revision questions and answers download PDF

Your content is always enlightening. Office Products