
THE EMPIRICAL FORMULA (o-level chemistry)

THE EMPIRICAL FORMULA
The empirical formula of a compound is the simplest formula, which shows the ratio of the atoms present in a compound or a molecule by mass.
The molecular formula of a compound is the formula, which shows the number of each kind of atoms present in the compound.
TO CALCULATE THE EMPIRICAL FORMULAE
Example I
Sodium sulphate has the following composition by mass; sodium 32.4% sulphur 22.5% and oxygen 45.1%.
solution

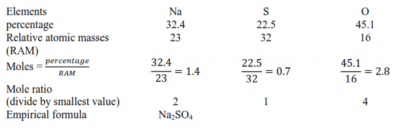
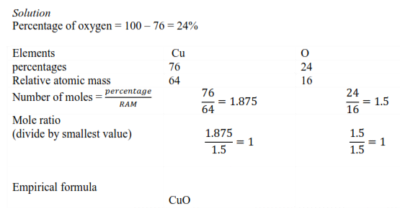
Example 2
A compound contains oxygen and copper only. The molecular mass is 159.0.
What is its Empirical and molecular formula if the percentage of copper is 76

For revision question and answers download the PDF
Sponsored by The Science Foundation College +256 753 80 27 09
Compiled by Dr. Bbosa Science
CATEGORIES O-level chemistry
TAGS Dr. Bbosa Science

Thank you very much
I have understood better
Thank you so much.
Good job
Thanks but I need some question papers
download from digitalteachers.co.ug website