
Electrochemical cells (A-level physical chemistry)

Electrochemical cells
When a strip of metal is placed in a solution of its ions, the metal atoms tend to ionize; cations dissolve in solutions while electrons remain on the metal surface. For instance, a divalent metal, M, ionizes as follows
M(s) ↔ M2+ (aq) + 2e ……………………………………………………………………………..(1)
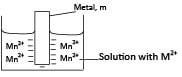
The attraction of the metal rod (containing negative charge) and the solution (containing positive charges) causes a potential difference called electrode potential.
The metal dipped into it’s a solution containing metal ions is called electrode half-cell represented symbolically as Mn+/M. For example, a half-cell of zinc rod dipped in zinc ion solution is symbolized as Zn2+/Zn
Definition
Electrode potential is a potential difference that is set up between a metal and its solution containing metal ions.
Factors affecting the magnitude of the potential difference
- The concentration of metal ions already in solution. Since the ionization equation (1) is a reversible reaction, the higher the concentration of metal ions in solution, the further to the left will be the state of equilibrium and hence the smaller the electrode potential.
- Temperature. Increasing temperature increases the ionization of metal atoms and thus increases the electrode potential.
- Pressure: applicable to gas systems, the higher the pressure the higher the electrode potential because increased pressure increases the gas concentration at gas-liquid junction.
- Position of metal in electrochemical series. Metals above hydrogen in electrochemical series, have ionization reactions that lie more to the right than left, thus, the metal tends to be negatively charged with respect to their solutions. Thus, have negative electrode potentials.
Metals below hydrogen in electrochemical series, their ionization reaction lie more to the left and their electrode potentials are positive.
Standard electrode potential (SEP)
This is the electrode potential of a metal dipped in a solution containing one mole of metal ions in a dm3/litre of solution at 298K and 1 atmosphere. It is denoted by E0.
Factors affecting standard electrode potential
Electrode potential involves formation of hydrated ions. It is a sum of atomization energy (which is endothermic) + ionization energy (which is endothermic) + ionic hydration energy (which is exothermic).
When atomization and/or ionization energy is high, the standard electrode potential becomes more positive.
On the other hand, when the hydration energy is higher than the sum of ionization and atomization energy, standard electrode potential is negative.
Short-hand Notation for an electrode half cells
A metal rod dipped in a solution of metal ions constitutes a half cell. The accepted short form of writing an electrode is to write is to write ‘oxidized form/reduced form’ of metal. For example, the zinc half-cell is written as Zn2+/Zn and copper electrode as Cu2+/Cu.
Standard Hydrogen electrode (fig. 4.2)
It consists of hydrogen gas at 298K and pressure of 1 atmospheres bubbling over a strip of platinized foil (i.e. platinum coated) in a solution which is 1M with respect to H+ ions

The shorthand notation for a hydrogen electrode is
H+, M/ ½ H2, 1 atm. Or H+/ ½ H2.
And electrode reaction is
H+(aq) + e → ½ H2(g)
A potential develops on the surface of the platinum; by convention, it is assigned an arbitrary value of zero volts.
Hydrogen electrode is use as a reference electrode and the electrode potential of all other electrodes are measured relative to this.
Measurement of electrode potential of metal (fig. 3)
A standard metal electrode is combined with a standard hydrogen electrode as shown in Fig.4.3 below

The two compartments in the figure are connected by a salt bridge. This contains an electrolyte such as potassium chloride, which conducts electricity but does not allow mixing of two solutions in half cells. The measurement on the high resistance voltmeter is the standard electrode of the metal since that of hydrogen half-cell is zero.
Definition
Standard electrode potential is the electrode potential value of an electrode measure with respect to a standard hydrogen electrode of 0 volts. When an electrode is immersed or dipped into a solution of 1M concentration of its ions at a standard temperature of 298K and pressure of 1 atmospheres.
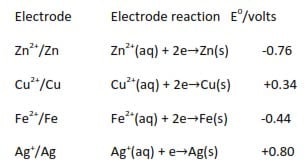
Some electrode and their standard electrode potentials are given below:
Galvanic or Voltaic cells or electrochemical cells
This is a type of cell in which a chemical reaction results in production of an electric current.
By combining two suitable electrodes (half cells) a cell of a particular emf may be obtained. One electrode acts as electron supplier and the other as electron acceptor.
The Daniel cell (fig. 4.4)
It consists of the zinc electrode dipped into 1M zinc sulphate solution and copper electrode dipped into 1M copper sulphate solution, the two solution separated by a porous partition. The cell develops an electromotive force (emf) of 1.10V.

Half call reaction
At zinc electrode (anode)
Oxidation occurs and the electrode dissolves.
Zn(s) – 2e → Zn2+(aq)
At the copper electrode
Reduction occurs (cathode)
Cu2+(aq) + 2e → Cu(s)
Overall equation
Zn(s) + Cu2+ (aq) → Zn2+ (aq) + Cu(s)
Cell notation
The cell in fig. 4 can be represented as
Zn(s)/ZnSO4 (aq)//CuSO4 (aq)/Cu(s)
Or simply
Zn(s)/Zn2+ (aq)//Cu2+ (aq)/Cu(s)
The single vertical line (/) indicates a phase boundary and double vertical lines (//) indicate a porous partition between the solutions.
Emf of a cell
The emf of the above cell is given by
E = ECu – Ezn
Rules regarding electrode potentials
- If the direction of an electrode reaction is reversed, the sign of its electrode potential must also be reversed.
Zn2+(aq) + 2e →Zn(s) E0 = -0.76V
Zn(s) → Zn2+ (aq) + 2e E0 = +0.76V
(b) If an electrode reaction equation is multiplied by a positive factor. The electrode potential must not be multiplied by that factor. It remains unchanged.
Zn2+(aq) + 2e →Zn(s) E0 = -0.76V
2Zn2+(aq) + 4e →2Zn(s) E0 = -0.76V
Not 1.52V
Generally the emf of a cell is defined as follow
E0 cell = E0R.H.E – E0L.H.E
NB. For a positive cell emf, R.H.E is for the half cell with most positive electrode potential.
Example 1
Calculate the standard emf of Zn/Zn2+//Cu2+/Cu from the data given below. Write down the half cell reactions and cell reaction.
Electrode E0/V
Zn2+/Zn -0.76
Cu2+/Cu +0.34
Answer
E0cell = E0R.H.E – E0L.H.E
= 0.34 –(-0.76) = +1.10V
Electrode reactions
At R.H.E (cathode)
Cu2+ (aq) + 2e→ Cu(s)
L.H.E (anode)
Zn(s) – 2e → Zn2+
Cell reaction
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Implication of the emf of a cell
For electrode; since, standard electrode potentials are reduction potentials, a negative value for E0 implies that the reduction reaction is non spontaneous while a positive value implies that the reduction reaction is spontaneous.
For a cell a negative value of E0Cell indicates that the cell reaction as written is non spontaneous and instead is spontaneous in the reverse direction.
Therefore, the value of standard electrode potential may be used to predict the direction of spontaneous chemical reaction. Then when writing down or constructing a cell given two electrodes together with their standard electrode potentials, the electrode with the less negative E0 (if both are negative or electrode with more positive value if both are positive) must be the right hand electrode of the cell and the other the left hand electrode.
Example 2
(a) Construct a cell using Li+/Li and Mg2+/Mg electrodes,
given that E0Li+/Li = -3.04V and E0Mg2+/Mg = -2.37V.
(b) Calculate the E0cell.
(c) Give the cell reaction
Solution
- Li(s)/Li+//Mg2+(aq)/Mg(s)
- E0cell = E0R.H.E – E0L.H.E
= -2.37 – (-3.04)
= +0.67V
- Cell reaction
2Li(aq) + Mg2+(aq) → Li+(aq) + Mg(s)
Standard electrode potential and standard free energy change
There is a relationship between electrochemistry and thermochemistry. In the electrode reaction
Mn+ (aq) + ne ↔ M(s)
If the standard free energy change, ∆G0 then the value of the standard electrode potential, Eo, at the same temperature is given by
∆G0 = -nFE0
Where n is the number of electrons transferred in the electrode reaction and F is the Faraday constant.
For example, E0 = +0.34V at 298K for the reaction
Cu2+(aq) + 2e → Cu(s)
The value of ∆G0 is given by
∆G0 = -2 x 96500 x (-0.34)
= 65.6kJmol-1.
Liquid junction potential
Is a potential difference set up across a phase boundary between two solutions in a cell. It tends to oppose the cell potential i.e. it reduces it and hence the observed cell emf is less than the actual emf of the cell

Causes of liquid junction potential
It is caused by the differential diffusion of charges across the porous partition between the two solutions of the cell. For example, in the Daniel cell, the Zn2+ ions diffuse into the CuSO4 solution and Cu2+ diffuses into the ZnSO4 solution.

The speed of migration of Zn2+ and Cu2+ cations across the phase boundary is different due to a number of factors. Some of which are either the ionic size and/or the extent to which they are hydrated. Due to this, a p.d. is set up across the boundary called Liquid Junction Potential.
This liquid junction potential may be eliminated by connecting the solutions in the two half cells by means of a salt bridge which is a glass tube containing a saturated solution of either KCl or NH4NO3.
NB. In the cell diagram/notation, the double lines either represent a porous partition or a salt bridge.
Types of electrodes
- The metal/metalloid e.g. Zn2+/Zn
- The gas electrode e.g. Pt, H+/ ½ H2
- The metal/insoluble electrode: this type of metal coated with one of its insoluble salt surrounded by a solution containing the anion of the salt e.g. Hg/Hg2Cl2, KCl(aq) or Hg/Hg2Cl2, Cl– This electrode is commonly known as a calomel electrode.
- Ag/AgCl(s), Cl–
Possible electrode reactions
As R.H.E (reduction)
AgCl (s) + e → Ag(s) + Cl–(aq)
As L.H.E (oxidation)
Ag(s) + Cl–(aq) → AgCl(s) + e
Hg/Hg2Cl2, KCl(aq) or Hg/Hg2Cl2, Cl– This electrode is commonly known as a calomel electrode.
Possible electrode reactions
As R.H.E (reduction)
Hg2Cl2(s) + 2e → 2Hg(l) + 2Cl–(aq)
As L.H.E (oxidation)
Hg (s) + 2Cl– (aq) – 2e → Hg2Cl2(s)
The two electrodes Ag/AgCl(s), Cl– and Hg/Hg2Cl2, KCl(aq) have stable potential and are not easily affected by oxidants or reducing agents, and because of their high stability, they are sometimes used as reference electrodes since hydrogen electrode in practice is easily poisoned by oxidants and reducing agents.
- Oxidation—reducing (redox) electrodes
This type comprises a reduced species and oxidized species in the same solution, e.g. Pt/Fe3+, Fe2+ or Pt/Cr3+, Cr2+.
Dry cells
These were invented to overcome the difficulty of electrolytic solution leaking out of cell such as Daniel cell. In dry cell, the electrolyte is made into a paste. An example is shown below
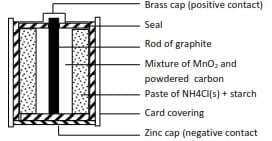
This type of cell is used in radio, flashlight and clocks as it is portable.
The initial electrode processes are
Anode:
Zn(s) → Zn2+(aq) + 2e
Cathode:
2NH4+(aq) + 2e → 2NH3(g) +2H2(g)
The lead-acid accumulator
The cell stores or accumulates electric charges. It consists of two lead plates dipping into a 30% solution of sulphuric acid. Both plate becomes covered with an insoluble film of lead II sulphate
First, the cell must be charged. A direct current is passed through the cell. The processes which take place are:
Charge
Positive plate
PbSO4(s) + 2e → Pb(s) + SO42-(aq)
Negative plate
The plates are now different and therefore have different potentials, so that, when they are connected, an electric current will flow between them. When the cell supplies an electric current, i.e. discharge the process which take place are:
Negative plate
Pb(s) + SO42-(aq) → PbSO4(s) + 2e
Positive plates
PbO2(s) + 4H+(aq) + SO42-(aq) + 2e → PbSO4(s) + 2H2O(l)
NB: the plate which is positive during the charge becomes negative during the discharge.
Watch this
For revision questions and answers download PDF
Electrochemical cells (A-level)

Generally, I enjoy the many examples and the videos in this website which widens my understanding. Dissection videos greatly increased my dissection knowledge. Self studies during this lockdown made easy using digitalteachers.co.ug. My vision of triple “A” shall come true, thank you very much Mr. Bbosa and all other teachers at large. I have admired Science Foundation College, I refer every student who wants his/her dreams to come true to visit digitalteachers.co.ug during this lockdown period.
wish you success
I fell in love with this website!
This’s one of the best websites