
Explain the variation of atomic radii in the first series of transition elements

Variation of atomic radii in transition elements
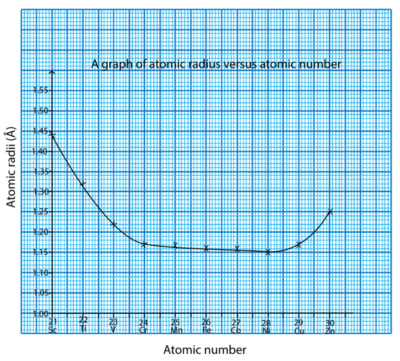
The atomic radii of elements of a particular series decrease gradually up to the midway element (from Sc to Mn) and then these values remain almost constant up to the elements of group 1B (Fe-Cu). The last element of each series i.e. Zn shows an increase in atomic radius.
Reason
For elements from Sc to Mn, the atomic radii decrease because of gradual increase in nuclear charge with increase in atomic number. From Fe to Cu, the atomic radii remain almost constant because electrons added to the d-orbital screen the 4s electron(s), and the attraction between the nucleus and 4s electrons decreases. The additional nuclear charge is counter balanced by the screening effect.
Towards the end of the series, there is an increase in electron-electron repulsion between the electrons added to 3d and 4s orbitals. This increase in repulsion becomes greater than the attraction between the nucleus and the 4s electrons, thus, because of the greater magnitude of electron-electron repulsion, the atomic size of Zn is greater than that of Cu.
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much

I have liked the notice,they are more brief and precised as compared to other notice on online.Please is it possible to find for Melting and boiling points of such metals and some clue on the complexis formed
This is a common misunderstanding of IPv6 You are not required to use global addresses in IPv6 If you have things which are local and which you would like to be local-only, you can use link local IPv6 addresses