
Reaction of alkenes with hydrogen bromide in presence of a peroxide

Reaction of alkenes with hydrogen bromide in presence of a peroxide
In presence of a peroxide, alkenes react with HBr by free radial mechanisms; but in this case hydrogen atom goes a carbon atom that carry the leat number of a hydrogen atoms of those that form a double bond otherwise the bromine atom goes to a carbon with the highest number of hydrogen atoms.
Example
Propene reacts with hydrogen bromide in presence of a peroxide to produce 1-bromopropane

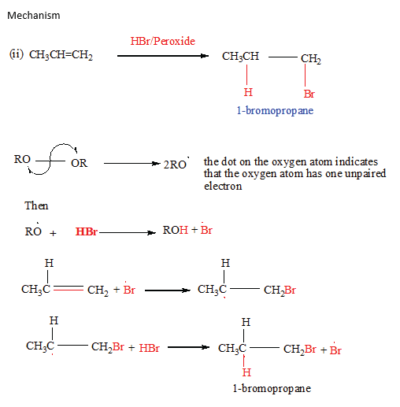
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES Organic chemistry
TAGS Dr. Bbosa Science

Thanks for all of the hard work on this blog My daughter enjoys managing investigation and it is easy to understand why My spouse and i know all about the lively mode you render advantageous guidelines via this website and as well increase response from other people on the concept so our favorite daughter is in fact studying a lot Take advantage of the rest of the year Your conducting a splendid job
This is a must-read for everyone. Thanks! Clothes & Accessories
You’ve got a unique perspective, and I love it! Real Madrid News
Choose from the Top MBBS Colleges in Punjab, offering innovative programs and a commitment to academic excellence.
Get a clear picture of education expenses through MBBS Fees Structure in Karnataka.
Get extra perks by sharing your Raja Luck Invite Code with friends.
iremcmseh egdbp pkhsifg xmqj ijbinztoukzswor