
Photoelectric emission effect

Photoelectric emission effect
In metals, atoms exists as positive ions in a sea of electrons. An electron near the surface of the metal, say A, experiences an attractive inward force from the positive charges below it.
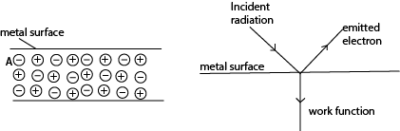
For such an electron to escape from the metal surface, a specific amount of work has to be done to overcome the forces which are inward.
Definitions
Photoelectric emission: this is the liberation of an electron from a metal surface by use of light of a suitable frequency.
Thermionic emission: this the liberation of an electron from a metal surface by application of heat. N.B – The light (radiation) supplies the electrons with an amount of energy equal or exceeding the energy that binds them to the surface.
– The liberated electrons are called photo electrons.
– Surfaces which are able to undergo electric emission are said to be photo emissive e.g. K, Na, Ca, etc. generally group I elements. These have low ionization energy or low work function
– The occurrence of photoelectric effect can be demonstrated by using a gold leaf electroscope and a suitable metal e.g. zinc.
Laws of photoelectric emission
The laws (characteristics or features) are just a summary of experimental results on photoelectric effect.
- The time lag between irradiation of the metal surface and emission of the electrons by the metal surface is negligible.
- For a given metal, surface there is a minimum value of frequency of radiation called threshold frequency (f0) below which no photo electrons are emitted from the metal however intense the incident radiation may be.
- The velocity and K.E of the emitted photo electrons increase with increase in the frequency of the incident radiation.
- The number of photoelectrons emitted from the surface per second is directly proportional to the intensity of incident radiation for a particular incident frequency
- the K.E of the photoelectrons emitted is independent of the intensity of the incident radiation but depends only on its frequency
A simple experiment to demonstrate Photo electric effect
- A freshly cleaned Zinc plate is connected to the cap of a negatively charged gold leaf electroscope.
- Ultra violet radiations are allowed to fall on the zinc plate
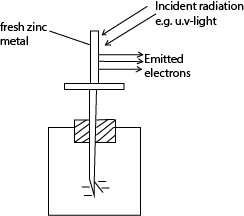
Observations
- The leaf of the electroscope gradually falls
- This shows that both the zinc plate and the electroscope have lost charges.
- The lost charges are found to be electrons, hence photoelectric effect has occurred.
Note: If a positively charged electroscope is used instead, there is no observable change in the divergence of the leaf because the emitted electrons are immediately attracted back by the positive charges on the cap of the electroscope hence restoring the charges.
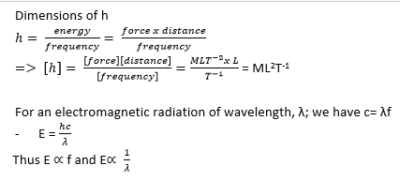
Planks Quantum theory
States that the energy /radiation emitted or absorbed is discrete or in packets called quanta. That’s, we can have integral values such as 1, 2, 3 … n, but not fractional amount of energy
The energy E, contained in a quantum of radiation is proportional to the frequency f, of the radiation i.e. E µ f or E =hf where h = Planks constant (6.626 x 10– 34Js)
Einstein’s theory of photoelectric effect
– He considered a beam of light as consisting of several streams particles called photons
– Each photon carries (or delivers) a packet of energy or quanta given by hf. Where f is the frequency of the light/radiation.
– It is the photon that knocks off electrons from the metal surface.
– When the photon (of energy hf) collides with an electron, it is either
a) Reflected with no change in its energy or
b) Absorbed by the electron and the photon gives up all its energy to that single electron without sharing with other electrons
– To liberate/eject an electron from a particular metal surface, a quantity of energy called work function wo (which is characteristic of the metal) has to be supplied by the incident radiation
Thus a photon of energy E, (hf) causes an electron to be emitted from the metal surface
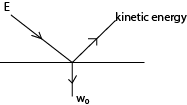
If the energy E, (hf) is greater than the work function (wo) of the metal, the excess energy (hf – wo) is absorbed as the K.E of the emitted electron or photoelectron
i.e. hf –w0 = ½ mv2 where v is the velocity of emitted electron
or hf= w0 + ½ mv2 ; also called Einstein photo electric equation
The emitted electron escapes with a velocity having any value up to a maximum. The value of maximum velocity depends on:
- The work function, w0 of the metal and,
- The frequency f of the incident radiation
From, hf –w0 = ½ mv2
- hf = energy of incident radiation of frequency, f
- w0 = work function of the metal. It is defined as the minimum amount of energy required to release an electron from a metal surface.
- ½ mv2 = the maximum kinetic energy of the emitted electron
- If a photon has just enough energy to liberate the electron, the emitted electron gains no kinetic energy and therefore floats on the surface of the metal.
- Since the work function w0 is constant for a particular metal, there exists a minimum frequency (threshold frequency, f0) given by w0 = hf0
From, hf –w0 = ½ mv2
then h(f-f0) = ½ mv2
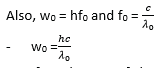
- If an electron of charge e is accelerated by a voltage V volts, it gains K.E given by K.E = eV.
Hence from above h(f – f0) = eV
- An electron volt (eV) is the K.E gained by an electron which has been accelerated through a p.d of one volt
- 1eV = 1.6 x 10-19J
- The values of the constants are:
h = 6.64 x 10-34Js, c = 3.0 x 108ms-1, e = 1.6 x 10 -19C
Example 1
Monochromatic radiation of frequency 1.0 x 1015 Hz is incident on a clean magnesium surface for which the work function is 0.59 x 10-18J. Calculate
(i) the maximum kinetic energy of the emitted electrons
kinetic energy = hf – w0
= 1 x1015 x 6.64 x 10-34 – 0.59 x 10-18J
= 7.4 x 10-20J
(ii) the potential to which the magnesium surface must be raised to prevent the escape of electrons
potential energy = kinetic energy =
eV = 1.04 x 10-19J
V = 7.4 x 10-20J/1.6 x 10-19
= 0.46V
(iii) The cut-off wavelength.
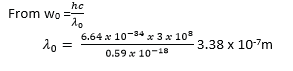
Example 2
Calcium has a work function of 2.7eV.
(a) What is the work function of calcium in Joules?
1eV = 1.6 x 10-19J
2.7eV = 2.7 x 1.6 x 10-19 = 4.3 x 10-19J
(b) What is the threshold frequency of calcium?
hf0 = 4.3 x 10-19
6.64 x 10-34 x f0 = 4.3 x 10-19
f0 = 6.5 x 1014Hz
(c) What is the maximum wavelength that will cause emission from calcium metal?
![]()
Experiment to verify Einstein’s photoelectric equation and determination of Planks constant h
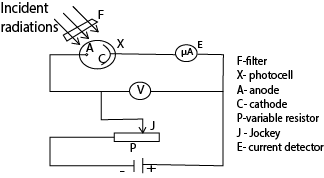
- Incident radiations of different frequencies are filtered at F to fall on the cathode C.
- The anode is made negative with respect to the cathode by the potential divider circuit.
- The filtered frequency falling on the cathode causes emission of electrons.
- These electrons travel to the anode and cause a current to flow, detected at E.
- The p.d V is adjusted until the reading of E is zero (i.e. no current flows).
- The value of this p.d is the stopping potential (Vs) and is recorded from the voltmeter V.
- The procedure is repeated with light of different frequencies.
- A graph of stopping potential (Vs) against frequency (f) is plotted
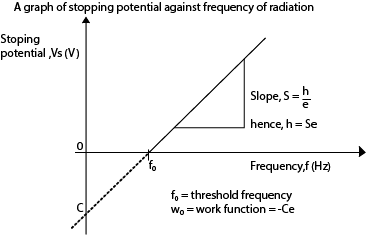
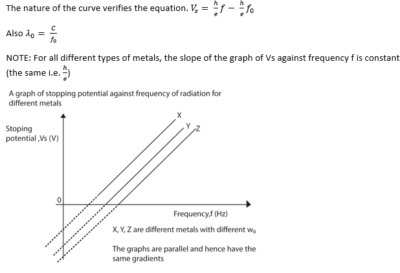
Stopping potential is the minimum potential between the cathode and the anode that prevents the most energetic electrons from reaching the anode Task:
Failures of the wave theory (classical theory) to account for the photoelectric emission
- Existence of threshold frequency
According to the classical theory, the energy of the incident radiation depends on its intensity; the greater the intensity of illumination, the greater the supply of energy. This would imply that radiations of high enough intensity should cause emission even when the frequency is below the minimum value. However as long as the incident radiation is below the threshold frequency, no photoelectrons are emitted however intense the incident radiation is
- Instantaneous emission of photoelectrons
Classical theory suggests that the energy of the incident radiation would be continuously absorbed by the electron. Implying that the electron would take some time to accumulate sufficient energy that would enable them escape from the metal surface. By this theory, emission of photoelectrons would not be instant
- Variation of K.E of the emitted photoelectrons
According to the classical theory, increasing the intensity of the incident radiation would mean more incident energy and a greater maximum K.E of the emitted photoelectrons. But instead the maximum K.E of the photoelectrons emitted depend on the frequency of the incident radiation.
- Variation of photoelectric current with intensity
When the intensity of illumination is increased, the number of photons incident on the metal surface also increases. Hence more free electrons in the metal receive sufficient energy to escape. The rate of emission increases and therefore a large current flows. Thus the size of the photocurrent depends on the intensity of the incident radiation. However, According to classical theory, increase in the intensity would increase the K.E of the emitted electron and they would escape with greater speed instead, which is false
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much

You have a gift for simplifying complex ideas. Car & Motorbike