
Explain the effect of increase in temperature on the saturated vapour pressure of a liquid.

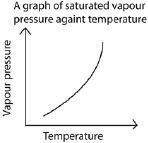
The saturated vapor pressure of any substance depends on its temperature. At higher temperatures, more molecules have sufficient kinetic energy to break from the liquid surface into the vapor phase. Under these conditions, equilibrium is reached at a higher pressure.
Please obtain free notes, exams and marking guides of Physics, chemistry, biology, history, economics, geography … from digitalteachers.co.ug website.
Thanks
Dr. Bbosa Science
CATEGORIES General
TAGS Dr. Bbosa Science
