
Alcohols (A-level organic chemistry)

Alcohols/Alkanols
These are organic compounds that contain at least one hydroxyl group (-OH) attached to saturated carbon atom.
Classification
(a) According to the number of –OH group
(i) Monohydric alcohols have one hydroxyl (-OH) group
Example
CH3CH2OH ethanol
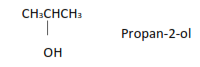
(ii) Dihydric alcohols or glycols have two hydroxyl groups Examples

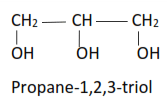
(iii) Trihydric alcohols have three hydroxyl groups.
(iv) Polyhydric alcohols or polyol contain more than three hydroxyl groups
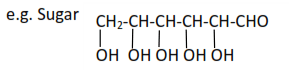
(b) Classification of monohydric alcohols
They are classified according to the number of alkyl groups attached to the carbon atom that bears a hydroxyl (OH) group.
(i) Primary alcohols have one alkyl group attached to the carbon atom that carries OH group, i.e. ROH.
Example
CH3CH2OH Ethanol
CH3CH2CH2OH Propan-1-ol
(ii) Secondary alcohol: have two alkyl groups attached to the carbon atom that bears OH group, i.e. R2CHOH
Example

(iii) Tertiary alcohols: have three alkyl groups attached to a carbon atom that bears OH group
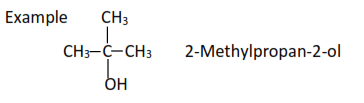
Nomenclature
(a) Are named by replacing the final “e” in the corresponding alkanes with “ol”
(b) The hydroxyl group is taken as a substituent group, and its position is given by numbering the carbon atoms in the chain from the side nearest to the carbon atom that carries OH group.
Examples
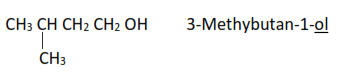
Physical properties
- Alcohols have high melting and boiling points than corresponding hydrocarbon of similar molecular masses due to intermolecular hydrogen bonds. consequently alcohols ate either liquids or solids.
Example
Propane (44) Bpt = -420C
Ethanol (46) Bpt = 780C
2. Lower members are completely soluble in water due to the formation of hydrogen bonds with water. But solubility of alcohols decrease as alkyl group length increase due to increase in “alkane like” character.
Preparation of alcohols
(a) Industrial preparation
(i) From petroleum products,e.g. alkenes
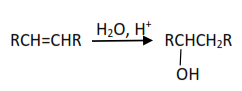
(ii) Fermentation of sugars
Ethanol can be made by fermentation of sugars. Fermentation is usually carried out by adding yeast to a mixutre of sugar and water.

(iii) Methanol is produced by catalytic reduction of carbon monoxide.
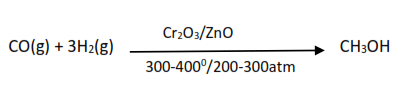
(b) Laboratory preparation alcohols
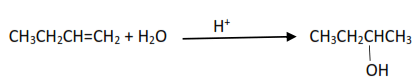
(i) Hydration of alkenes in presence of acid catalyst
(ii) By hydrolysis of alkyl halides using aqueous alkali

(iii) By reduction of carbonyl compounds (aldehyde and ketones) using lithium aluminium hydride (LiAlH4) in presence of dry ether.
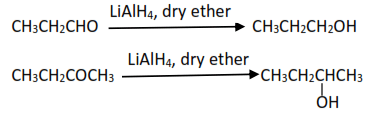
(iv) Reduction of carboxylic acids using LiAlH4 in dry ether

Absolute ethanol
All aqueous solutions of ethanol yield, on fractional distillation a constant boiling mixture (azeotrope) of 96% ethanol and 4% water known as rectified spirit.
(i) In the laboratory rectified spirit is stored over quick lime overnight. Quick lime dehydrates the mixture; then pure ethanol called absolute ethanol is distilled.
(ii) In industry, benzene is added to the rectified spirit. Distillation yields three fractions
At 650C a constant boiling mixture of ethanol, benzene and water.
At 680C a constant boiling mixture of ethanol and water
At 780C pure ethanol distills of
Reactions of monohybrid alcohol
- Cleavage of O-H bond
- Cleavage od C-O bond
- Oxidation
- Dehydration
(a) Cleavage of O-H bond
(i) Behavior as a weak acid
2ROH + 2Na → RO–Na+ + H2(g)
Acidity: primary alcohol> secondary alcohol>tertiary alcohol
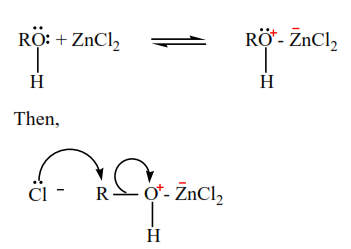
(ii) Behavior as a base
ROH + H+ → RO+H2
(iii) Formation of ether
Ethanol reacts the presence of concentrated sulphuric acid at 1400C to form diethyl ether.

(iv) Formation of esters from alkylhalides
(a) Alcohols react with carboxylic acids in the presence of a mineral acid (phosphoric or sulphuric acid) to form esters. However, this is not a good method because the reaction is reversible and does not go to completion.
Example
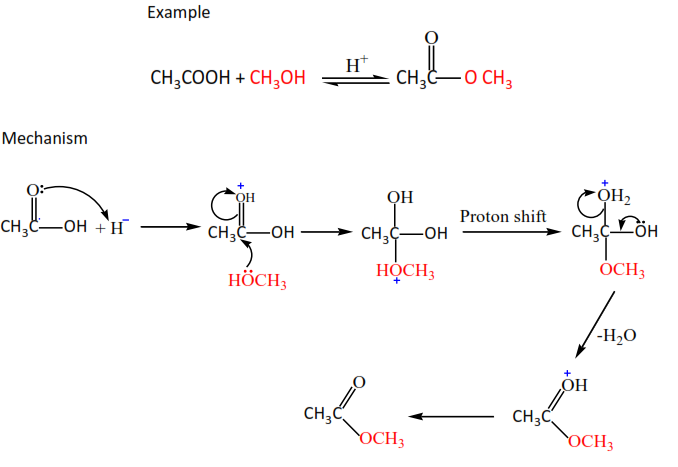
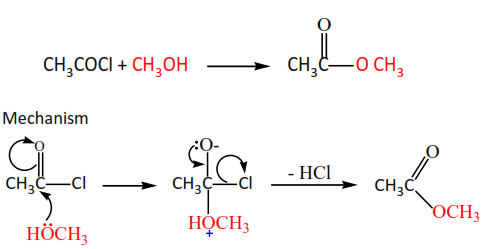
(b) Alcohols react with acid halides to form esters.
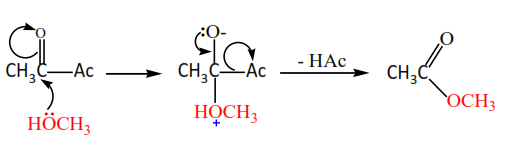
(c) Alcohols react with acid anhydride to form esters.

Mechanism
(b) Cleavage of C-O bond
Formation of alkyl halide
Alcohols react with a variety of reagents to yield alkyl halides. The most commonly used reagents are hydrogen halides (HCl, HBr and HI) phosphorus tribromide (PBr3) and thionyll chloride.
Examples
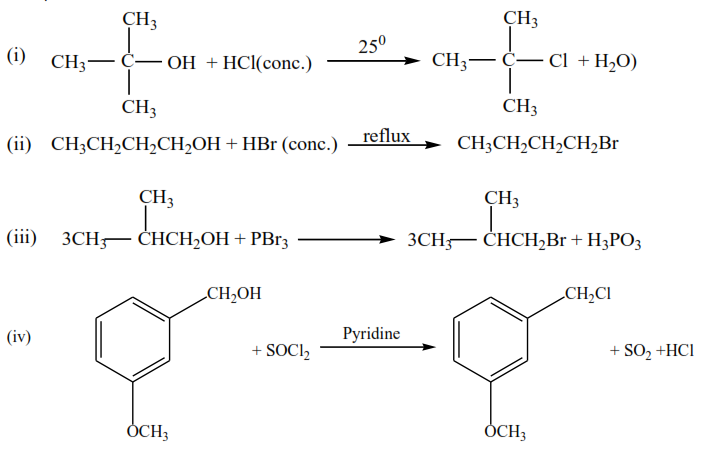
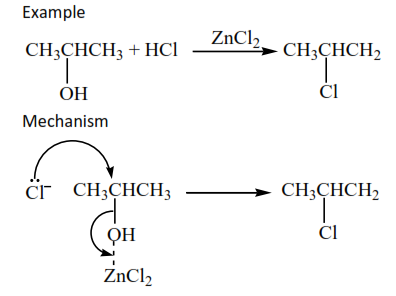
The order of reactivity of hydrogen halides is HI> HBr> HCl whereas the order of reactivity of alcohols is 30> 20> 10. The reaction of HCl with alcohol is catalyzed by anhydrous zinc chloride. The reaction is used to distinguish between primary, secondary and tertiary alcohols.
Tertiary alcohol react readily with HCl in presence of anhydrous zinc chloride to form an insoluble chloride giving two layers immediately.
Secondary alcohols form two-layer in 5 -10minutes
Primary alcohol does not form layers at room temperature.
Mechanism
Example
(c) Oxidation
(i) Alcohols are burnt in oxygen to produce carbon dioxide, water, and heat. Due to the production of heat on combustion, alcohol is used as fuel.
(ii) Mild oxidizing agents like acidified potassium dichromate oxidize primary alcohols to aldehydes and then, to carboxylic acids. Secondary alcohols are oxidized to ketones.
(iii) Tertiary alcohols are not oxidized by potassium dichromate and therefore acidified potassium dichromate is used to distinguish tertiary alcohols from primary or secondary alcohols. When reacted with primary or secondary alcohol, the color of acidified potassium dichromate changes from orange to green.
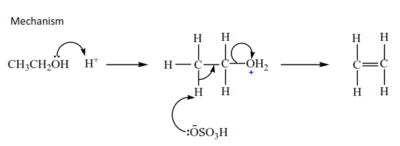
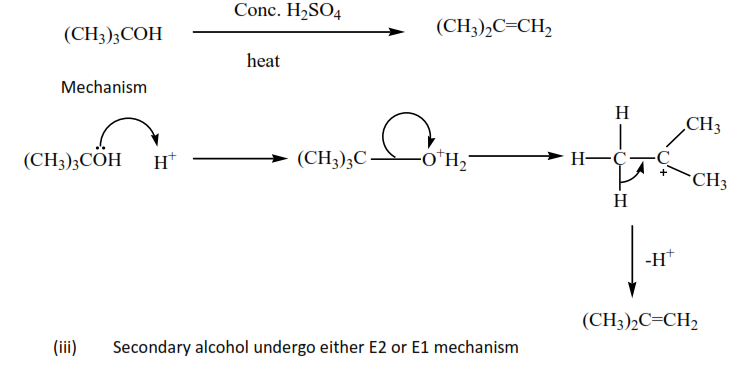
(d) Dehydration of alcohol
Alcohols are dehydrated by hot concentrated sulphuric or phosphoric acid to form alkenes. The mechanisms depend on the class of alcohols.
(i) Primary alcohol undergoes elimination bimolecular; E2.
Example



(ii) Tertiary alcohols undergo elimination unimolecular; E1.
Example
Watch this
Sponsored by The Science Foundation college +256 753 80 27 09
Compiled by Dr. Bbosa Science
Thanks

nice learning lessons so resourceful
I think this is one of the most important information for me. And i am glad reading your article. But wanna remark on some general things, The website style is wonderful, the articles is really excellent : D. Good job, cheers
Woah I’m just genuinely digging the style/thought of this web site. It’s straightforward, however fantastic. Additional frequently than not it is demanding to acquire the perfect involving exceptional usability along with visual physical appearance. I should say you have carried out a brilliant task. In addition, your web page starts tremendous rapidly personally with Web explorer. Outstanding Internet site
Naturally I like your web-site, but you have to take a look at the spelling on quite a few of your posts. A number of them are rife with spelling issues and I find it very bothersome to tell you. On the other hand I’ll surely come back again!
Hi my family member! I want to say that this post is awesome, great written and include almost all significant infos. I’d like to peer more posts like this .
Thankyou for this wondrous post, I am glad I observed this website on yahoo. [Reply]
It??s nice to definitely locate a blog the spot that the blogger is . Appreciation for making your blog site.
Document Article. Was’t whatever i ended up specifically hunting for however My husband and i did some searching Aol your posting showed up i really tested versus eachother and additionally wished to no less than thanks a ton.
Hey, you used to write fantastic, but the last few posts have been kinda boring… I miss your great writings. Past several posts are just a bit out of track! come on!
Hey admin, thank you very much for providing this blog post. I found it superior. Cheers, Taj!
you can buy some promise rings from ebay but those are the cheap ones, the quality ones are sold elswhere..
cheers for taking the time to discuss this, I feel strongly about it and love learning more on this topic. If possible, as you gain expertise, would you mind updating your blog with more info? as it is extremely useful for me.
I found your website through a random stroke of luck. It helped me do my research on this topic. Its amazing to think one site could contain so muck information. You are doing the great work!
i like the role of Anthony Hopkins in the movie Silence of The Lambs. this guy is simply amazing.,
I’m extremely impressed with your writing talents as smartly with the format for your blog. Is this a paid topic or did you modify it yourself? Either way keep up the nice high quality writing, it is rare to look a great blog like this one these days.
I am often to blogging and i also truly appreciate your content. Your content has really peaks my interest. Let me bookmark your web site and maintain checking for brand spanking new information.
You made some decent points there. I looked on the internet for the issue and found most people goes along with with your site.
every woman loves to wear those pretty but expensive diamond rings, i would love to give my girlfriend a diamond ring”
Very interesting read, you should look into some Online Advertising
the air compressors that we use at home are the high powered ones, we also use it for cleaning~
oprah also makes some good book reviews, i always wait for the book reviews of oprah.
Thank you for this kind of information I was looking around all Yahoo to discover it!
An impressive share, I given this onto a colleague who was simply performing a little analysis within this. And the man the truth is bought me breakfast simply because I discovered it for him.. smile. So ok, i’ll reword that: Thnx for your treat! But yeah Thnkx for spending time to go over this, Personally i think strongly concerning this and adore reading more about this topic. If you can, as you become expertise, do you mind updating your blog with an increase of details? It truly is extremely helpful for me. Large thumb up with this blog post!
I want reading through and I conceive this website got some really utilitarian stuff on it! .
Woah this is just an insane amount of information, must of taken ages to compile so thank you so much for just sharing it with all of us. If your ever in any need of related info, perhaps a bit of coaching, seduction techniques or just general tips, just check out my own site!
Thanks for your type info.. I realy appreciate it.. keep it up.
I’m impressed, I have to admit. Genuinely rarely can i encounter a blog that’s both educative and entertaining, and without a doubt, you’ve hit the nail for the head. Your notion is outstanding; the thing is something inadequate folks are speaking intelligently about. My business is very happy that we found this within my seek out some thing in regards to this.
Your blog is one of a kind, i love the way you organize the topics.”:*,,
Good day, May I download the photograph and employ it on my personal web site?
Hello, you used to write fantastic articles, but the last several posts have been kinda lackluster… I miss your super posts. Past few posts are just a little bit out of track!
I like this post plenty. i’ll undoubtedly be back. Hope that ready to be able to scan additional insightful posts then. are sharing your data with all of my friends!
|Tato stránka má rozhodně všechny informace, které jsem o tomto tématu chtěl a nevěděl jsem, koho se zeptat.|Dobrý den! Tohle je můj 1. komentář tady, takže jsem chtěl jen dát rychlý
Your content is consistently valuable. Computer & Accessories
I learned something new today. Thank you! Indian Cricket
The Top MBBS Colleges in Gujarat are renowned for their comprehensive medical programs.
Learn how to secure a medical career through MBBS Admission Through Management/Nri Quota in Punjab.
Obrigado|Olá a todos, os conteúdos existentes nesta
Good day! Do you know if they make any plugins to assist with Search Engine Optimization? I’m trying to get my blog to rank for some targeted keywords but I’m not seeing very good gains. If you know of any please share. Appreciate it!
Hello there! I know this is kinda off topic but I’d figured I’d ask. Would you be interested in exchanging links or maybe guest authoring a blog post or vice-versa? My blog addresses a lot of the same subjects as yours and I feel we could greatly benefit from each other. If you might be interested feel free to shoot me an email. I look forward to hearing from you! Great blog by the way!
buď vytvořil sám, nebo zadal externí firmě, ale vypadá to.
|Hello to all, for the reason that I am actually keen of
I’m not sure why but this blog is loading extremely slow for me. Is anyone else having this problem or is it a issue on my end? I’ll check back later and see if the problem still exists.
værdsætter dit indhold. Lad mig venligst vide det.