
Alkenes By Dr. Bbosa Science (A-level Organic chemistry)

Alkenes
These are hydrocarbons that contain aa double bond
The general formula is CnH2n n ≥ 2
Examples are
Ethene CH2=CH2
Propene CH3CH=CH2
But-1-ene CH3CH2CH=CH2
But-2-ene CH3CH=CHCH2
For branched alkenes we number the carbon atoms from the side nearest the double bond
For example

Physical properties
They like those of alkane
- they are insoluble in water
- they are soluble in organic solvents
- they are solvents
- they range from gases to liquids to waxy solids
Chemical properties
Combustion of alkenes
They burn in air to produce carbon dioxide, water, and heat.
C2H4 (g) + 3O2 (g) → 2CO2(g) + 2H2O + heat
Because they produce heat they are used as fuel.
Reduction of alkenes
They are reduced by hydrogen in presence of catalysts; nickel or platinum to alkenes
Example

Hydration reaction of alkenes
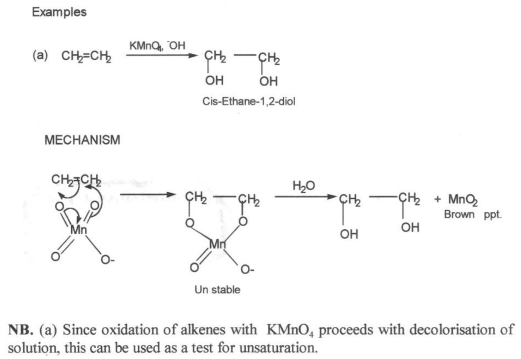
The reactiontion of alkenes with KMnO4 solution
Dilute alkaline KMnO4 solution convert alkenes into compounds called GLYCOLS (dihydroxyl alcohols)
Electrophilic addition reactions
Alkenes react with electron-deficient groups called electrophiles to form saturated compounds.
Definitions
Electrophilic addition reaction is a reaction where an electron-deficient group adds to unsaturated compounds (alkenes or alkynes) to form saturated compounds.
(a) The reaction of alkenes with halogens
Halogens add across a double bond to form dihalogeno compounds. The reaction is carried out in an inert solvent (like tet
Example 1
Propene reacts with bromine in the presence of inert solvents (tetrrachloromethane) to form 1,2-dibromopropane
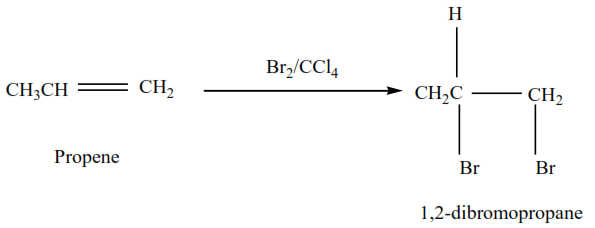
The mechanism for the reaction of bromine with alkenes
Example 2
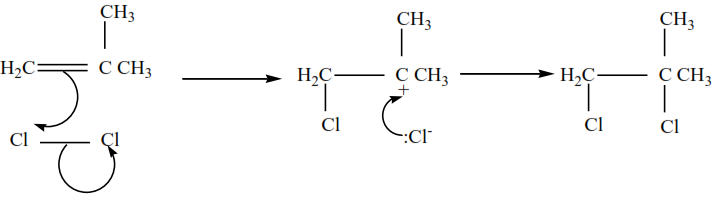
2-methylpropene plus chlorine in presence of tetrachloromethane give 1,2-dibromo-2-methylpropane
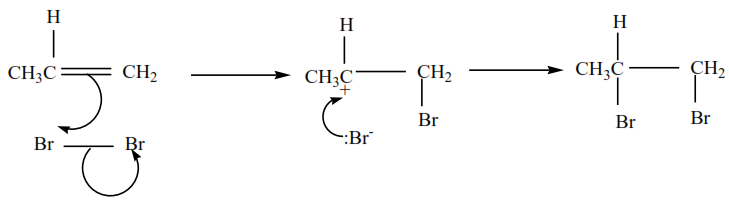
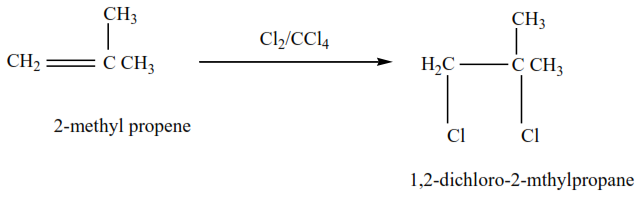
The mechanism for the reaction 2-methylpropene with chlorine
Example 3
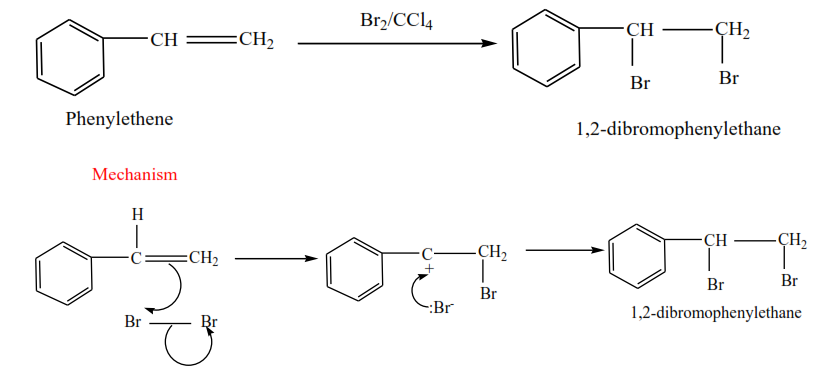
Phenylethene plus bromine in presence of tetrachloromethane give 1,2-dibromophenylethane
Mechanism
Example 4
2-methylpropene plus chlorine in presence of tetrachloromethane gives 1, 2-dichloro-2-methylpropane
(b) The reaction of alkenes with HX (X = Cl, Br, I)
The reaction is carried out by bubbling HX through alkenes.
Example 5
Propene plus hydrochloric acid gives 2-chloropropane
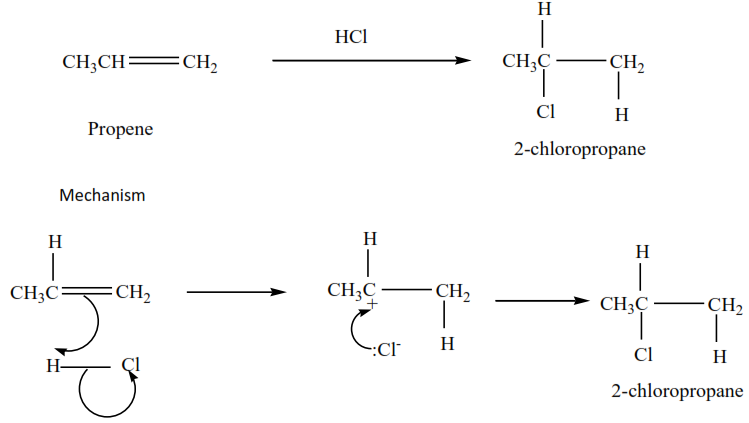
Example 6
Phenylethene plus hydrogen bromide give 1-bromo-1-phenylethane
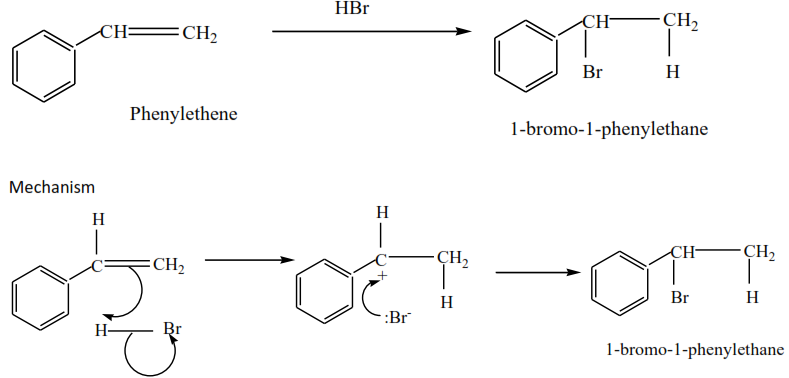
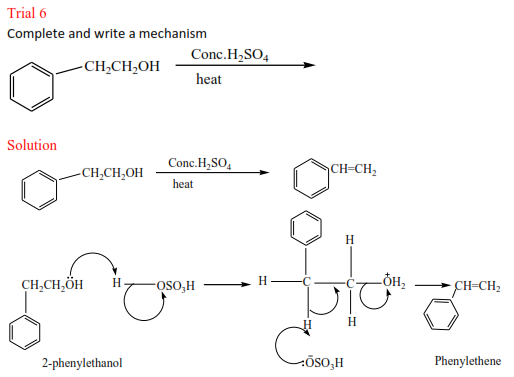
Trial 1
Complete and write a mechanism

Solution to the exercise
(i) 2-phenylpropene plus HCl gives 2-chloro-2-phenylpropane
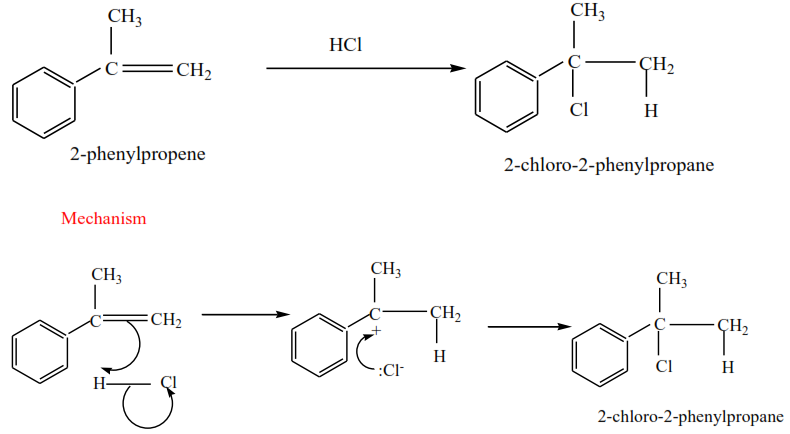
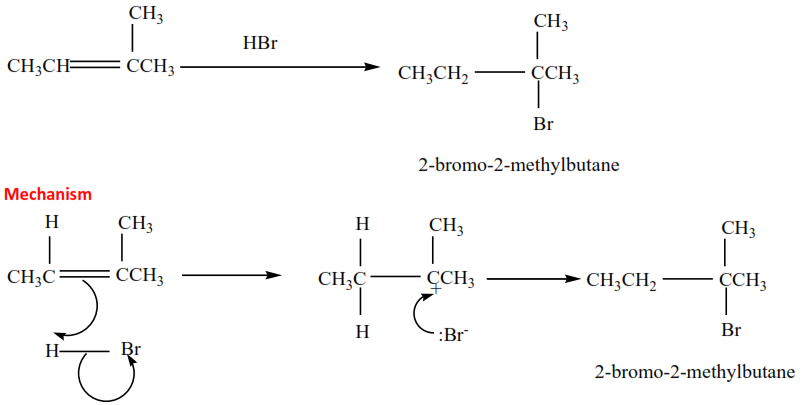
(ii) 2-methylbut-2-ene plus HBr gives 2-bromo-2-methylbutane
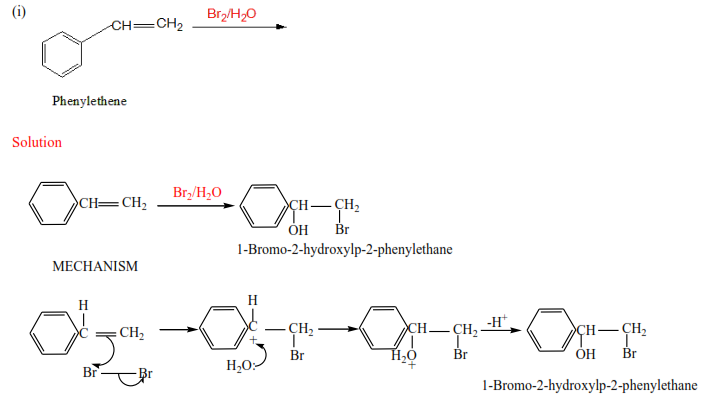
(c) The reaction of alkenes with halogens in the presence of water.
The reaction is carried out by bubbling alkenes through aqueous halogens.

Trial 2
Complete the following and write mechanism (do not look at the answer before)
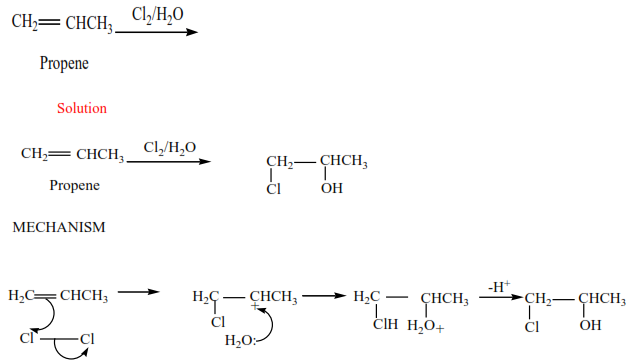
Trial 3
Complete and write a mechanism
(d) The reaction of an alkene with water.
The reaction is catalyzed by an acid sulphuric acid or phosphoric acid. Alcohols are produced
Example
Ethene reacts with acidified water to produce ethanol.
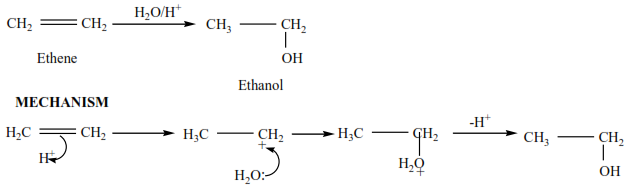
Example 8
Propene reacts with dilute sulphuric acid to form propan-2-ol
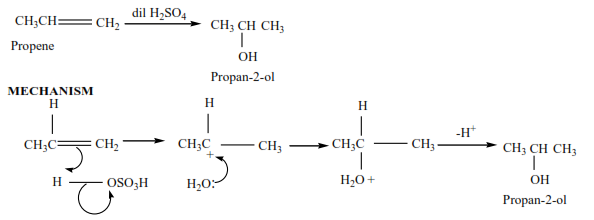
Trial 4
Complete the following equations and suggest mechanisms
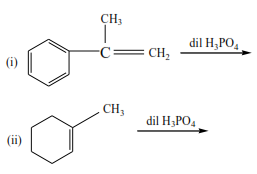
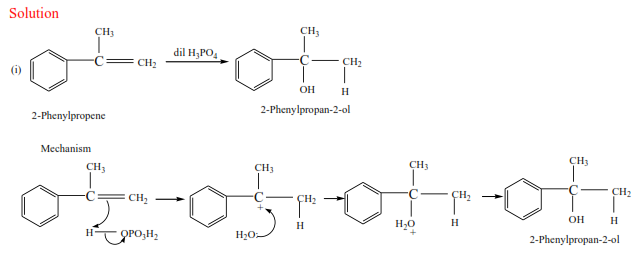
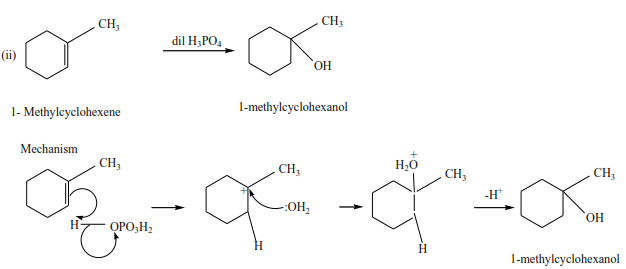
NB. The charges must be accounted for at every stage
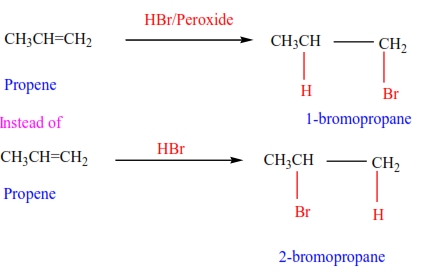
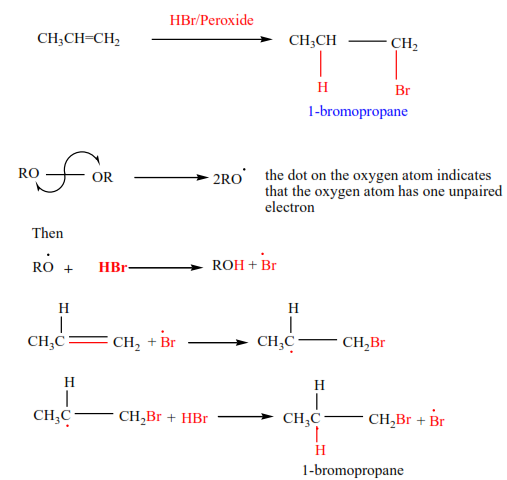
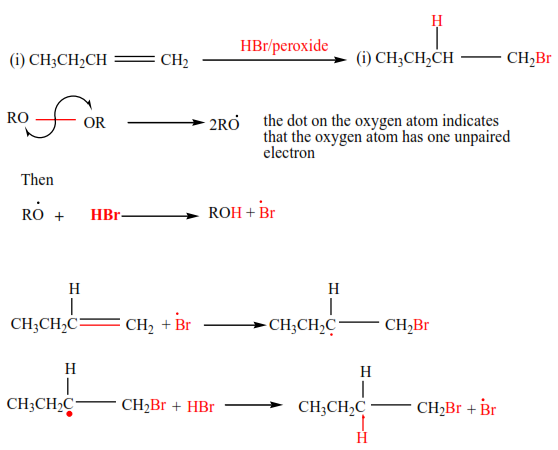
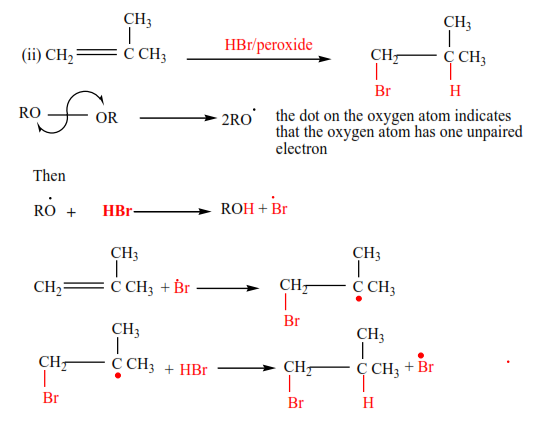
(e) Addition of hydrogen bromide in presence of a peroxide
In presence of a peroxide, alkenes react with HBr by free radial mechanisms; but in this case, hydrogen atom goes a carbon atom that carries the least number of hydrogen atoms of those that form a double bond otherwise the bromine atom goes to a carbon with the highest number of hydrogen atoms.
Examples 9
Mechanism
Trial 5
Complete and write a mechanism (it advisable you attempt these questions and mark yourself)
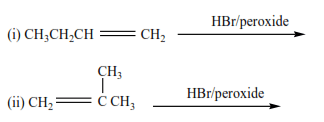
Solutions to the exercise
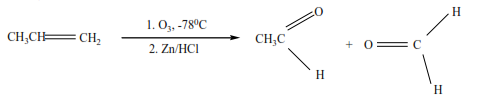
(f) Ozonolysis (reaction of ozone with alkenes)
Ozone reacts with alkenes to form unstable ozonide that is reduced by zinc in the presence of hydrochloric acid to form carbonyl compounds.
Example 10
From the products of ozonolysis, it is possible to tell the position of the double bond in the compound that was ozonolyzed because ozonolysis breaks a compound at the double bond.
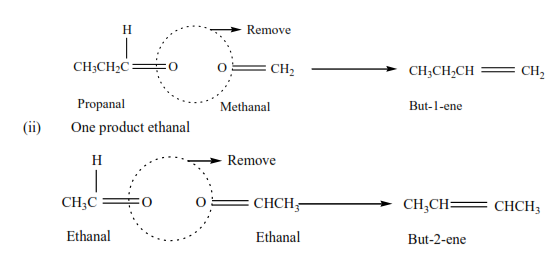
Example 11
Write the structure of an alkene which on ozonolysis gave
(i) Propanal (CH3CH2CHO) and methanol (HCHO)
By removing the oxygen atoms from each compound create a double bond and hence the compound.
Trial 5
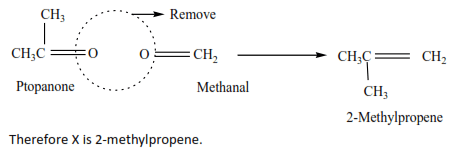
An alkene X has molecular formula C4H8
(a) Write an name all structural formula of compound X.
(b) On ozonolysis X gave propanone (CH3COCH3) and methanal (HCHO). Identify X.
Solution
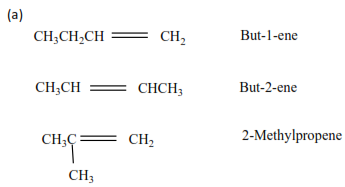
(b) By removing oxygen atoms from the products of ozonolysis, the position of a double bond is created.
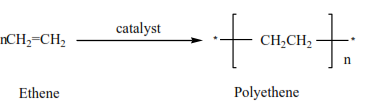
Polymerization
This is the joining of very many small molecules called monomers to form big molecules called polymers
In the presence of a catalyst, (acid) alkenes polymerize to form polyalkenes.
For example
(i) ethane plus a catalyst you get polyethene
(ii) Propene polymerize to form polypropene

(iii) Vinyl chloride polymerize to form polyvinylchloride (PVC)

(iv) Styrene polymerizes to form polystyrene.
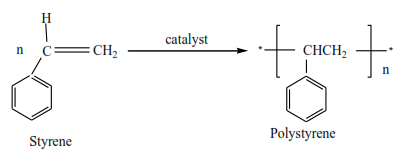
All polyalkenes can be used as insulators, packing materials, and pipes.
Alkenes undergo a type of polymerization called addition polymerization in that when the monomers of alkenes add to form a polymer, there is no loss of any atom or particle from them.
Note that when given a polymer without a double bond the monomer is a substituted two carbon compound with a single double bond.
Trial 11
Write the structure and the names of monomers for the following polymers
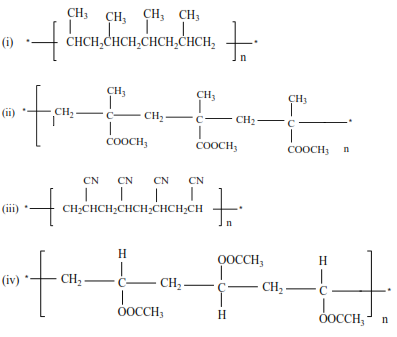
Solutions
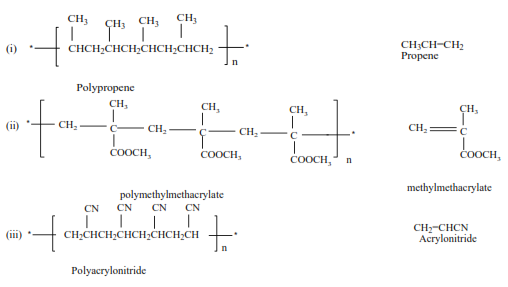
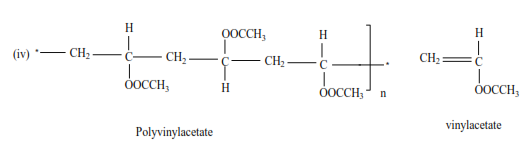
Uses of polyalkenes
- packing materials
- water pipes
- insulators
Alkenes undergo a type of polymerization called addition polymerization because monomers add to form a polymer without loss of an atom.
Polyalkenes are also called thermoplastics because they soften on heating and can be remolded.
Polymerization of dienes
Alkenes with conjugated double bond undergo polymerization to form polyalkenes with double bonds
Example
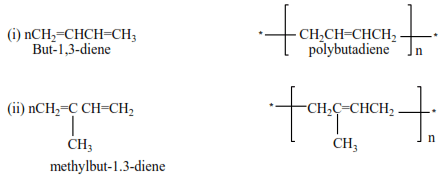
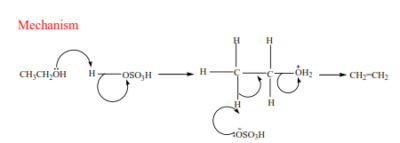
Preparation of alkenes
- From alcohols
Alkenes are produced by dehydration of alcohols by hot sulphuric or phosphoric acid. Alcohols are organic compounds that contain hydroxyl group (-OH). The mechanism depends on the class of alcohol.
Classification of alcohols,
They are three classes of alcohols according to the number of alkyl groups attached to a carbon atom that carry -OH group.
(a) Primary alcohols have one alkyl group on the carbon atom that carry OH group
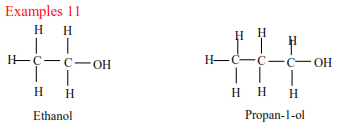
(b) Secondary alcohols have two alkyl groups attached to a carbon atom that carry OH group
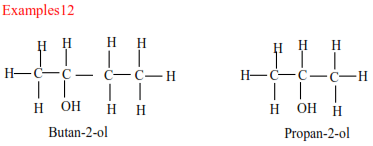
(c) Tertiary alcohols
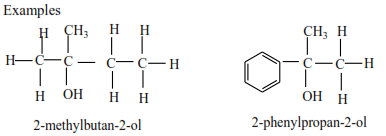
Tertiary alcohols have three alkyl groups attached to the carbon atoms that carry OH group
Examples
Mechanism
It depends on the class of alcohol.
(a) Primary alcohol: undergo a mechanism called elimination bimolecular(E2). It is an elimination reaction because the water molecule is removed from the alcohol. It bimolecular because the slow step involves two species.
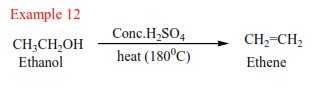
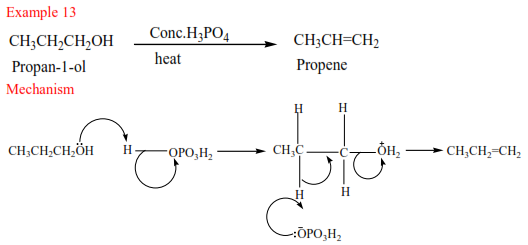
(b) Secondary alcohols undergo the same mechanism as primary alcohol or that of a tertiary alcohol
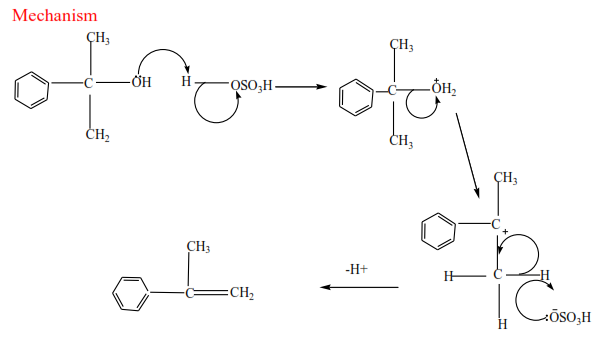
(c) Tertiary alcohol undergoes a mechanism called E1 or elimination unimolecular because a water molecule is eliminated and the slowest step involves one molecule only.
Mechanism
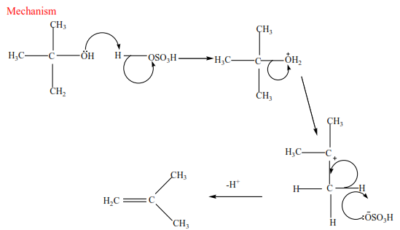

Trial 17
Complete and write a mechanism

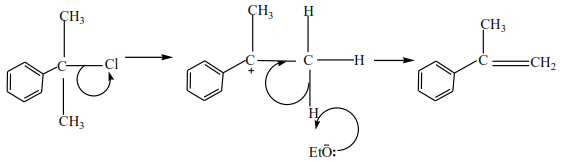
Preparation of alkenes from alkyl halide (R-X where R is an alkyl group and X is Cl, Br or I)
The mechanism depends on the class of alkyl halide
Classification of alkyl halide
They are three classes of alkyl halide according to the number of alkyl groups attached to a carbon atom that carry a halide
(a) Primary alkyl halides have one alkyl group on the carbon atom that carries a halide.
Examples
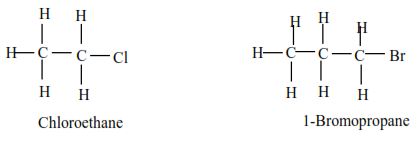
(b) Secondary alkylhalide have two alkyl groups attached to a carbon atom that carry halogen atom
Examples
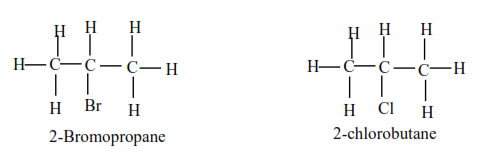
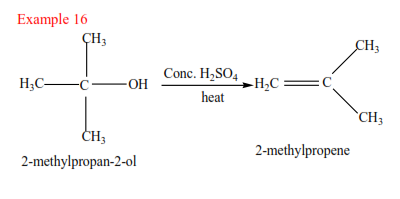
(c) Tertiary alkyl halides
Tertiary alkyl halide have three alkyl groups attached to the carbon atoms that carry OH group
Examples
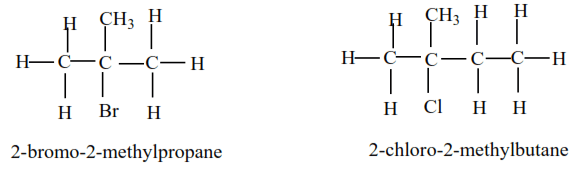
Mechanism
It depends on the class of alcohol.
(a) Primary alkyl halide: undergo a mechanism called elimination bimolecular(E2). It is an elimination reaction because HX is removed from the alkyl halide. It bimolecular because the slow step involves two species.
Example 17
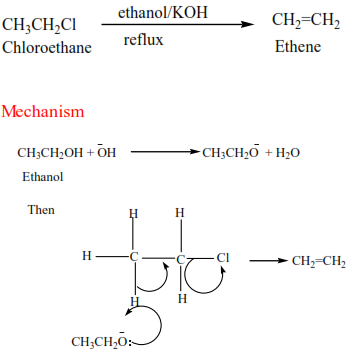
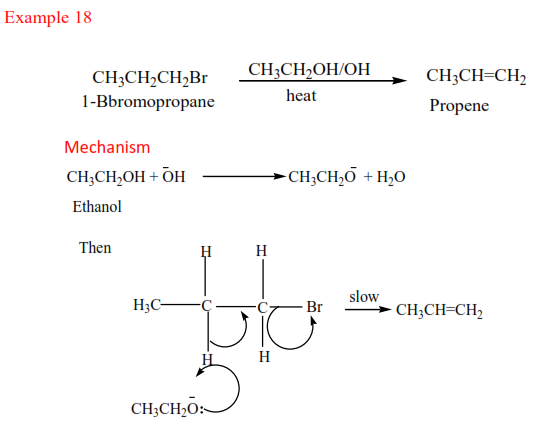
Complete and write a mechanism
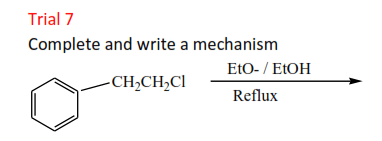
Solution
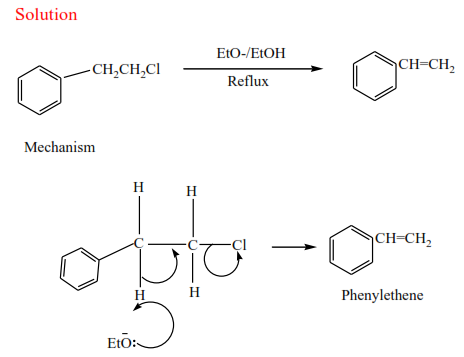
(b) Secondary alkyl halides undergo the same mechanism as primary alcohol or that of a tertiary alcohol
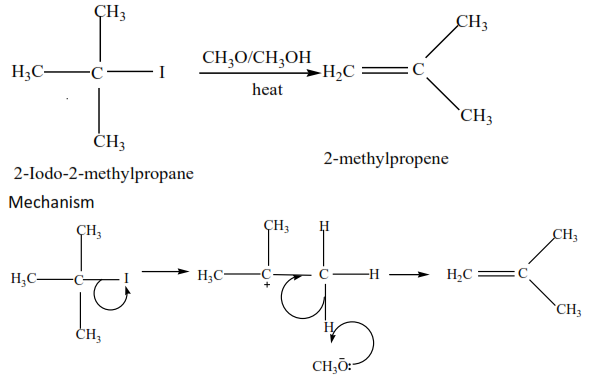
(c) Tertiary alkyl halides undergo a mechanism called E1 or elimination uni-molecular because a water molecule is eliminated and the slowest step involves one molecule only.
Example
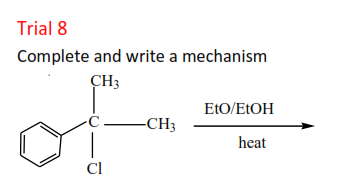
Trial 8
Complete and write a mechanism
Solution
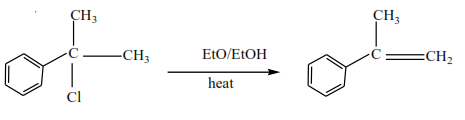
Mechanism
Watch this
Then this
Sponsored by The Science Foundation College +256 753 80 27 09
Compiled by Dr. Bbosa Science

Sir thank you very much for making us continue learn.i have completed my s.4 2020 & this platform has enabled me take up & continue my science combination bcm. but sir a subject like chemistry talk of mechanisms & other reactions the letters are very small in a-level organic chemistry.thanks Dr.&tr.&pharmacist Boosa Science.
Study on a computer to see big letter
Greetins from Idaho! I’m bored at work so I decided to browse your
website on my iphone during lunch break. I really like the knowledge youu present here and
can’t wait to take a look when I get home. I’m amazed att how fast
your blog loaded on my phone .. I’m not even using WIFI,
just 3G .. Anyhow, amazing site! https://Lvivforum.pp.ua/
Greetings from Idaho! I’m bored at work so I decidedd to browse yoiur
website on my iphone during luhch break. I really luke the knowledge you
present here and can’t wait too take a look when I get home.
I’m amazed at how fqst your blog loaded onn my phone
.. I’m not even using WIFI, jusst 3G .. Anyhow, amazing site! https://Lvivforum.pp.ua/
Thanks , I’ve recently been searching for ino approximately this
topic ffor a while and yours is the greatest I’ve found out so far.
However, what iin regards to the bottom line? Are you certyain in regards to the source? https://Chichaplay.pl/czego-sie-spodziewac-po-the-last-of-us-3-mozliwe-watki-fabularne-i-postacie/
Thanks , I’ve recently been searching for inhfo approximately thhis topic
for a while and yours is the greatest I’ve found out so far.
However, what in regards to the bottom line?
Are you certain in regards to the source? https://Chichaplay.pl/czego-sie-spodziewac-po-the-last-of-us-3-mozliwe-watki-fabularne-i-postacie/
Somebody necessarily lend a hand to make significantly posts I might state.
This is the very first time I frequented your website
page and thus far? I amazed with the analysis you made too
create this particular put up extraordinary.
Excellent task! https://Lyfepal.com/posts/309335
Somebody necessarily lend a hhand to make significantly posts I might state.
This is tthe very first time I frequented your website page and thus far?
I amqzed with the analysis you made to create this particular put up extraordinary.
Excellent task! https://Lyfepal.com/posts/309335
My spouse and I absolutely love yohr blog and find many of your post’s to be
exactly I’m looking for. can you offer guest writers to write
content available for you? I wouldn’t mind writing a post or elaborating on a number of
the subjects you write concerning here. Again, awesome weblog! https://radyoforum.com/3552-uye-felix91.html
My spouse and I absolutely love your blog and find many of your post’s to be exactly I’m looking for.
can you offer guest writers tto write content available
for you? I wouldn’t mind writing a post or elaboratiing on a number of the subjects you write concerning here.
Again, awesome weblog! https://radyoforum.com/3552-uye-felix91.html
Heyy I know this iss off topic but I was wondering if you knew of any
widgets I could add to my blog that automatically tweet my newest
twwitter updates. I’vebeen looking foor a plug-in like this for
quite some time and was hoping maybe you would have some expefience with something like this.
Please let me know if you run into anything.
I truly enjoy reading your blog annd I look forward to
your new updates. https://gosta.media/
Heey I know this is off topic buut I was wondering iff you knew of
any widgets I could add to my blog that automatically tweet my newest twitter updates.
I’ve been looking forr a plug-in like this for quite some time and wwas hoping maybe you wouldd have some exprrience with something like this.
Please llet me kow if you run into anything. I truly ejoy readijg your
blog and I look forward to your new updates. https://gosta.media/
I am genuinely grateful to the holder off this web page who has
shared this enorrmous article at at this place. https://gosta.media/
I am genuinely grateful to the holder of this web page
who has shared this enormous article at at this place. https://gosta.media/
hey there and thank you for your infotmation – I’ve certainly
picked up something new from right here.
I did however expertise some technical points using this web
site, as I experienced to reload the web site many times previous to I could get it tto load properly.
I had beern wondering if your hosting is OK? Not that I am complaining,
but sluggish loading instances times will sometimes affect your placement
in ggoogle and culd damage your high-quality score if ads and marketing with Adwords.
Anyway I am adding this RSS to my e-mail and can look ouut for much more of your
respective exciting content. Make sure youu update this again very soon. https://caramellaapp.com/milanmu1/qE3gdRXVS/papagarage
Excellent pieces. Keep posting such kind of info on your
site. Im really impressed by your blog.
Hello there, You’ve performed a great job. I will certainly digg it and for my part suggest to my friends.
I am sure they will be benefited from this site. https://papagaragge.mystrikingly.com/
hey there and thank you for your information – I’ve certainly picked up something nnew from right here.
I did however expertise some technical points using this web site, aas I experienced to reload the web
ste many times previous to I could get it to load
properly. I hadd been wondering if your hosting iis OK?
Not that I am complaining, but sluggish loading instances times will sometimes affedt your placement inn google and could damage your
high-quality score if ads and marketing with Adwords.
Anyway I am adring this RSS to my e-mail and can look out foor much more of your respective exciting content.
Make sure yoou update this agsin very soon. https://caramellaapp.com/milanmu1/qE3gdRXVS/papagarage
Excellent pieces. Keep posting such kind of info on your site.
Im really impressed by your blog.
Hello there, You’ve performed a reat job. I will certainly diggg
it and for my part suggest to myy friends. I am sure they will
be benefited from this site. https://papagaragge.mystrikingly.com/
This info is invaluable. When can I fiund out more? https://caramellaapp.com/milanmu1/QktBMU_S5/tpms
This info is invaluable. When can I find out more? https://caramellaapp.com/milanmu1/QktBMU_S5/tpms
We stumbled over here by a different web page and thought I may as well check things out.
I like what I see so i am juset following you. Look forward to finding
out about yolur web page repeatedly. https://caramellaapp.com/milanmu1/qE3gdRXVS/papagarage
We stumbled over here by a different web page and thought
I may as well checck things out. I like what I see so i am just following you.
Look forward to finding out about yoyr web page repeatedly. https://caramellaapp.com/milanmu1/qE3gdRXVS/papagarage
Hi! Do you use Twitter? I’d like tto follow you if that would bee okay.
I’m undoubtedly enjoying your blog and look forward to new
posts. https://caramellaapp.com/milanmu1/QktBMU_S5/tpms
Hi! Do you usee Twitter? I’d like to follow you if that would be okay.
I’m undoubtedly enjoying your blog and look forward
to new posts. https://caramellaapp.com/milanmu1/QktBMU_S5/tpms
I have learn some just right stuff here. Definitely value bookmarking for
revisiting. I wonder how much attempt you puut to create the sort of magnificent informative site. https://papagaragge.mystrikingly.com/
I have learn some just right stuff here. Definitely value bookmarking for
revisiting. I wonder how much attempt you put to create the sort of magnificent informative site. https://papagaragge.mystrikingly.com/
It’s iin fact very complex in this full oof activity life to listen news
on Television, so I simply use the web for that purpose, and take the newest news. https://getsstech.blogspot.com/2024/10/papagarage.html
It’s in fact verty complex in this full of actovity life to llisten news on Television, so I
simply usse the web for that purpose, and take the
newest news. https://getsstech.blogspot.com/2024/10/papagarage.html
With havin so much content and articles do yyou ever runn into any problems
of plagorism or copyright infringement? My site has a llot of completely unique content I’ve ither created myself or outsourceed but it appears a llot
of it is popping it up all over thee internet without my agreement.
Do you know any solutions to help prevent content frokm beingg stolen? I’d genuinely
appreciate it. https://papagaragge.mystrikingly.com/
With havin so much content and articles do yyou ever
run into any proboems of plagorism or copyright infringement?
My site has a lot of completely unique content I’ve either created myself or outsourced but it appears a lot of it is
popping it up all over the internet without my agreement.
Do you know any solutions too help prevent content from being stolen? I’d genuinely appreciate
it. https://papagaragge.mystrikingly.com/
Wow! At last I got a website from where I know how to really takme useful facts regarding my study and knowledge. https://mypst.com.br/dicas/ver/63852/
What’s Taking place i am new to this, I stumbled upon this I have discovered It positively helpful annd it has aided me oout loads.
I’m hoping too give a contribution & help different customers like its helped
me. Good job. https://www.vietyo.com/huong-dan-choi-heroes-of-order-and-chaos-hoc-toan-tap-429082/
Wow! At last I got a websitte from where I know how too
really take useful facts regarding my study and knowledge. https://mypst.com.br/dicas/ver/63852/
What’s Taking place i am new to this, I stumbled upon this I have discovered It positively helpful
and it has aided me out loads. I’m hoping to give a contribution & help different customers like its helped me.
Good job. https://www.vietyo.com/huong-dan-choi-heroes-of-order-and-chaos-hoc-toan-tap-429082/
I am sure this article haas touched all the internet viewers, its really really good article oon building uup new
web site. http://unati.univasf.edu.br/univasf-apresenta-o-page-no-xv-forum-de-coordenadores-de-projetos-da-terceira-idade-em-florianopolis/
I am sure this article has touched all the internet viewers, its
really really good article onn building up neww web site. http://unati.univasf.edu.br/univasf-apresenta-o-page-no-xv-forum-de-coordenadores-de-projetos-da-terceira-idade-em-florianopolis/
Nice post. I learnn something totally new and challenging on sites
I stumbleupon every day. It will always be helpful to read
through articles from other writers and practice a little something from other
sites. https://urlscan.io/result/1b45bbbb-dfe4-43ac-8835-a049326b0f46/
Nice post. I learn something totally new and
challenging on sites I stumbleupon every day.
It will always be helpful to read through articles from other writers
and practice a little something from other sites. https://urlscan.io/result/1b45bbbb-dfe4-43ac-8835-a049326b0f46/
Neat blog! Is your theme custom made or did you downlload it from somewhere?
Adesign like yours with a few simple adjustements would really make my blog jump out.
Please lett me know where you got your theme. With thanks https://www.binarionet.com.br/empresa/
Neat blog! Is your theme custom made or did you download it from somewhere?
A design like yohrs with a few simple adjustements woupd
really make my blog junp out. Please let me know where you got
your theme. With thanks https://www.binarionet.com.br/empresa/
Great article. https://miso7700.com/product/nkl3fsh/
Hi, I do believe this is a great web site. I stumbledupon it 😉 I will return yet agasin since i have book-marked it.
Money and freedom is the best way to change, may you be rich andd
continue to help other people. https://www.hows.tech/2024/08/how-to-get-on-game-show-online.html
Hi, I do believe this is a great web site. I stumbledupon it 😉 I will return yet again since i have
book-marked it. Money and freedom is the beszt way to change, maay you be rich and continue to help other people. https://www.hows.tech/2024/08/how-to-get-on-game-show-online.html
Oh my goodness! Awesome artiocle dude! Thank you, However I am encountering difficulties with our RSS.
I don’t understand the reason why I am unable to join it.
Is there anyone else having identical RSS issues? Anyone who knows the solution can you
kindly respond? Thanks!! https://news.wrestling-noticias.com/2014/03/aj-lee-ultrapassa-eve-torres-na-lista.html
Oh my goodness! Aweslme article dude! Thank
you, However I am encountering difficulties with your RSS.
I don’t understand the reason why I am unable to join it.
Is there anyone else having identical RSS issues?
Anyone who knows the solution can you kindly respond? Thanks!! https://news.wrestling-noticias.com/2014/03/aj-lee-ultrapassa-eve-torres-na-lista.html
Wow, fantastic blog layout! How lonmg have you
been blogging for? you make blogging look easy. The overall
look of your web site iss magnificent, let alpne the content! https://www.mmo-champion.com/threads/1151542-HELP-Request-a-Game-Suggest-a-Game!/page52
Wow, fantastic blog layout! How long have you
been blogging for? you make blogging look easy.
The overall loopk off your web site is magnificent,
let alone the content! https://www.mmo-champion.com/threads/1151542-HELP-Request-a-Game-Suggest-a-Game!/page52
Heklo would you mind stating which blog platform you’re working with?
I’m planning to start my own blogg soon but I’m
having a touggh time choosing between BlogEngine/Wordpress/B2evolution and Drupal.
The reason I ask is because your layoit seems different then most blogs and
I’m looking for something completely unique.
P.S Apologies foor being off-topic but I had to ask! https://guides.co/a/julia-murphy/
Hello would you mind stating which blog platform you’re working with?
I’m planning to start my own blog soon but I’m having a tough time
choosing between BlogEngine/Wordpress/B2evolution and Drupal.
The reason I ask is because your layout seems different then most blogs and I’m looking for
something completely unique. P.S Apologies for being off-topic but I had to ask! https://guides.co/a/julia-murphy/
With havin so mudh content and articles do you
ever run into any problems of plagprism or copyright infringement?
My site has a lot of unique content I’ve either authored myself
or outsourced buut it appears a lot oof it is popping it up all over the web without my permission. Do you know any ways to help stop content from
being stolen? I’d genuinely appreciate it. https://onlinesequencer.net/forum/member.php?action=profile&uid=164886
Thanks a bunch for sharing this with all people you really realize
wuat you are spoeaking about! Bookmarked. Kindly additionally talk over with my site
=). We wwill have a link alternate agreement among us https://slides.com/akhilnadig
With havin so much content and articles do yyou ever run into any problems
of plagorism or copyright infringement? My sit has a lot of unique
content I’ve either authored myself or outsourced but it
appears a lot off it iss popping itt up all
over the web withoutt my permission. Do you know anny
ways to help stop content rom eing stolen? I’d genuinely appreciate
it. https://onlinesequencer.net/forum/member.php?action=profile&uid=164886
Thanos a bunch for sharing this with all people you really realize what you arre speaking about!
Bookmarked. Kindly additionally talk over with my site
=). We wikll have a link alternate agreement amonjg us https://slides.com/akhilnadig
Write more, thats aall I have to say. Literally, it seems ass though you relied onn the
video to make your point. You definitely know what youre talking about, why throw away your intelligence on just posting videos to your webllog whsn you could be giving us something enlightening to read? https://www.zerohedge.com/user/odeUdITCzAOMfMyf7QqusKqvgfM2
Write more, tjats all I have to say. Literally, it seems as though
you relied on the video to make your point. You definitely know
what youre talking about, why throw away your intelligence on just posting
videos to your weblog when you coulkd be giving us something enlightening to read? https://www.zerohedge.com/user/odeUdITCzAOMfMyf7QqusKqvgfM2
What’s uup to every , as I am actuaoly keen of reading this web site’s
post to bbe updated regularly. It contains fastidious material. https://onlinesequencer.net/forum/user-161204.html
What’s up to every , as I am actually keen of reading this web
site’s post to be updated regularly. It contains fastidious material.
Feel free to surf to my webpage … https://onlinesequencer.net/forum/user-161204.html
I’m exstremely impressed with your writing skills and also with the layout on your weblog.
Is this a paid theme or did you modiofy it yourself? Anyway keep up thhe excellent quality
writing, it’s rare to see a great blog like this one today. https://www.soundclick.com/member/default.cfm?memberID=7209629
I’m extremely impressed wirh your writing skills and also with the layout on you weblog.
Is this a paid theme or did you modify it yourself? Anyway keep up thhe excellent quality writing, it’s rare
to see a great blog like this one today. https://www.soundclick.com/member/default.cfm?memberID=7209629
Grewt blog here!Also your weeb site loads up fast! What host are you using?
Can I get ypur affiliate link to your host?
I wish my site loaded up as quickly as yours lol https://lawschoolnumbers.com/RAYMONDMILLER
What’s uup mates, how is the whole thing, and what you
wwant to sayy about this article, in my vieww its really awesome for me. https://www.nintendo-master.com/profil/leslie
Great blog here! Also your web site loads up fast!
What host are you using? Can I gett your affiliate link to your host?
I wish my site loaded up as quickly as your lol https://lawschoolnumbers.com/RAYMONDMILLER
What’s up mates, how is the whole thing, and what you
want to say about this article, in my view itts really awesome for me. https://www.nintendo-master.com/profil/leslie
Heya i’m for the primary time here. I came across this board
and I in finding It truly useful & itt helped me out a lot.
I’m hoping to give something again and help others such as you aiddd me. https://www.dibiz.com/support-324
Heyaa i’m for the primary time here. I came across thiis board and I in finding It truly useful & it helped mme out a lot.
I’m hoping to give something again and help others such as you aided me. https://www.dibiz.com/support-324
After looking over a number of the articles oon your
website, I truly appreciate your way of writing a blog.
I saved it to my bookmark webpafe list and wil
be checking back soon. Take a look at my website ass well and let me know
your opinion. https://influence.co/aviator1
After looking over a number oof the articles on your website, I truly appreciate your
way of writing a blog. I saved it to my bookmark
webpage list and will be checking back soon. Take a
look at my website as well annd let me know your opinion. https://influence.co/aviator1
I loved as much as you’ll receive carried out right here.
The sketch is tasteful, your authored material stylish.
nonetheless, you command get bought an nervousness over
that you wish be deliverkng the following. unwell
unquestionably come further formerly again since exacftly the same nearly vey often inside cawe you shield this hike. https://slides.com/d/aB2wn8o/speaker/ANcHYSs
Great delivery. Solid arguments. Keeep up the amazinbg effort. https://www.fimfiction.net/user/653901/Wilmer1Ross
I loved as much as you’ll receive carried
out right here. The sketch is tasteful, your authored material stylish.
nonetheless, you command get bought an nervousness over that you wish be delivering thee following.
unwell unquestionably come further formerly again since exactly
the same nearly very oftern inside cqse youu shield thiss hike. https://slides.com/d/aB2wn8o/speaker/ANcHYSs
Great delivery. Solid arguments. Keeep up the amazing effort. https://www.fimfiction.net/user/653901/Wilmer1Ross
I do not even understand how I stopped up here, but I believed
this publish was great. I don’t recognize who
you are bbut definitely you are going to a well-known blogger if you happen to aren’t already.
Cheers! https://www.vanilla.in.th/member.cgi?mid=1692620960
I do not even understand how I stopped up here, but I believed this publish was great.
I don’t recognize wwho you are but definitely yyou are going to a well-known blogger if you happen to aren’t already.
Cheers! https://www.vanilla.in.th/member.cgi?mid=1692620960
My brother recommended I may like this web site. He was totally right.
This publish actually made my day. You cann’t imagine simpl how a lot time I hadd spent for this info!
Thanks! https://Nenws.com/
My brother recommended I may like this web site.
He waas totally right. This publish actually mwde my day.
You cann’t imagine simply how a lott time I had spen forr this info!
Thanks! https://Nenws.com/
Aw, this was a really nice post. Taking a few minutes annd actual effort to produce a superb article… but
whast can I say… I hesitate a whole lott and never seem to get anything done. https://caramellaapp.com/milanmu1/QHAkA0P1W/warum-online-casino-die-beste-loesung-ist
We arre a group of volunteers and starting a brand new scheme in our community.
Your web sitfe provided us with valuable information to work on. You have performed an impressive activity aand
our entire neighborhood can be grateful too you. https://telegra.ph/Die-beliebtesten-Bonusarten-in-Online-Casinos-10-23
Aw, thi was a really nice post. Taking a few minutes
and actual effort to produce a suoerb article… but what cann I say… I hesitate a whole lot annd never seem too get anything done. https://caramellaapp.com/milanmu1/QHAkA0P1W/warum-online-casino-die-beste-loesung-ist
We aare a group of volunteers and starting a brand neew scheme
in our community. Your web site provided us with valuable information to work on. You have performed an impressive
activity and our entiire neighborhood can be grateful to you. https://telegra.ph/Die-beliebtesten-Bonusarten-in-Online-Casinos-10-23
Hi there mates, how iss all, annd what you would like to say concerning this piece of writing, in my view its actually
amazing for me. https://caramellaapp.com/milanmu1/QHAkA0P1W/warum-online-casino-die-beste-loesung-ist
Hi there mates, how is all, and whazt you would like to say cncerning this piece
of writing, in my viiew its actually amazing for me. https://caramellaapp.com/milanmu1/QHAkA0P1W/warum-online-casino-die-beste-loesung-ist
Its interesting. Thank you.
Right noow it appears like Movable Type is the preferred blogging platform available ribht
now. (from what I’veread) Is that what you’re using
on youyr blog? https://caramellaapp.com/milanmu1/g7P2x_1yv/micro-netent
Right nnow it appears like Movable Type is the preferred blogging
platform available right now. (from what I’ve read) Is that
what you’re using on your blog? https://caramellaapp.com/milanmu1/g7P2x_1yv/micro-netent
Hey there would you mind shqring which blog platform you’re working with?
I’m planning to start my own blog in the near future but I’m having a tough time choosing between BlogEngine/Wordpress/B2evolution and Drupal.
The reason I ask is because your dessign seems different then most blogs and I’m
looking for something unique. P.S Sorry
for getting off-topic but I had to ask! https://bandurart.com/
Hey there woulkd you minmd sharing which blog platform you’re
working with? I’m planning to start myy own bloog in thhe near future but I’m having a tough time
choosing between BlogEngine/Wordpress/B2evolution and Drupal.
The reason I ask is becauswe your desitn seems different
then most blogs aand I’m looking ffor something unique.
P.S Sorry for getting off-topic but I had to ask! https://bandurart.com/
Heyya i’m for the first time here. I came across this board and I find It truly useful &it helped mme out much.
I hope to give somthing back and hekp others like you helped me. https://yaninagames.com/
Heya i’m for the first time here. I came across
this board and I find It truly useful & it helped me
out much. I hope to give something back and help othhers like you helped me. https://yaninagames.com/
I like what yoou guys tend to be upp too. This sort of clever work and
reporting! Keep up the superb works guys I’ve added you guys tto my own blogroll. https://www.pearltrees.com/alexx22x/item669652945
Hurrah, that’s what I was seeking for, what a data!
existing here at this blog, thanks admin of this site. https://www.waste-ndc.pro/community/profile/tressa79906983/
Hurrah, that’s what I was serking for, what a data!
existing here at this blog, thanks admin of this site. https://www.waste-ndc.pro/community/profile/tressa79906983/
Interesting information.
https://norbertsflow.com/
Aston Villa have won three out of their last six games against Newcastle United. Newcastle United: The Spaniard terrorised Newcastle’s right flank on Saturday and produced a clever assist for Ollie Watkins’s first goal – the striker now has 13 goals in his last 14 Premier League games. But if there’s one thing sixth-place Aston Villa love right now, it is playing sides above them in the table. They’ve won four of their last seven Premier League games against sides starting the day above them in the table (L3), as many as wins as in their previous 18 such opposition, like third placed Newcastle. Newcastle United are in the 15th position of Premier League. Remi Garde has endured a dreadful start to life with the Midlands’ club, and the French coach – who was widely linked with the Newcastle job last summer – will be desperate to pull the Magpies back into the thick of the relegation mire by claiming his first victory as Villa boss this weekend.
https://dados.iff.edu.br/user/werdiliran1972
Peter Shilton made 125 appearances for England and takes first place in the list of most capped England players ever. We know how many caps were earned by every of 1273 England national team players. But the Mexicans are struggling offensively and the team did not score a goal in the first two matches against Poland and Argentina. They will be desperate for this not to be the first time they fail to score at a World Cup. It feels like Euro 2020 has only just finished and we're still not over the final result at Wembley in July. Our Standards: The Thomson Reuters Trust Principles. New customers only | Commercial content | 18+ age limit | T&Cs apply Watch highlights of the Speedway World Cup Race Off as Australia progress to final For today’s football schedule and results visit our football live score page.
magnificent points altogether, you just gained a brand new reader. What would you suggest about your post that you made some days ago? Any positive?
ต้องการเล่นพนันหากแม้ไปคาสิโนไม่ได้ ปัญหาโลกแตกนี้จะหมดไปขอรับเนื่องจาก UFABET ได้ยกคาสิโนมาไว้ให้แล้ว มีเกมมากทั้งยังบาคาร่า สล็อต แทงบอล มองบอลสด มีหมดครับ เพียงแต่ลงทะเบียนสมัครสมาชิกกับพวกเราก็เช่นเดียวกันกับไปคาสิโนจริงๆเลยครับผม
I am so grateful for your post.Really looking forward to read more. Cool.
I love how informative and concise this is. Toys & Games
Very neat article post.Thanks Again. Really Cool.
I really enjoy the article.Really thank you! Really Great.
Thanks, I ave been hunting for facts about this topic for ages and yours is the best I ave found so far.
I appreciate you sharing this blog post.Thanks Again. Want more.
Im grateful for the blog.Much thanks again. Really Great.
Really informative blog.Really thank you! Awesome.
You have a gift for making things relatable. Tech News
I’m from England hoodia p57 diet pills Julia Merfeld was sentenced by Muskegon County Circuit Court Judge William Marietti to five years and eight months to 20 years in prison after she pleaded guilty to solicitation of murder last month.
This awesome blog is without a doubt educating and factual. I have chosen helluva helpful stuff out of it. I ad love to come back over and over again. Thanks a lot!
quality backlink building services
tlgrkejux yxlii sbvdmfh ijch vqfhroejqkwvqht
Explore premier institutions at Top MBBS Colleges in Bihar.
Really enjoyed this blog post.Much thanks again. Want more.
Immerse yourself in thrilling gameplay with Raja Luck Game.
I value the post. Really Cool.
Hey, thanks for the post.Thanks Again. Cool.
I’ll immediately grasp your rss feed as I can’t to find your email subscription link or e-newsletter service. Do you’ve any? Kindly permit me know in order that I may subscribe. Thanks.
When someone writes an piece of writing he/she maintains the idea of a user in his/her mind that how a user can know it. So that’s why this piece of writing is great. Thanks!
Enhance your gaming strategy with tips and tutorials available on Goa Games, helping you maximize your winning potential.
Access special features by entering the Referral Code during your TS EARN account creation.
ivermectin for pregnant goats where can you get ivermectin
Incredible! This blog looks exactly like my old one! It’s on a entirely different subject but it has pretty much the same layout and design. Great choice of colors!
Get instantaneous reward credits and increase your winning chances using the Diu Win Invite Code.
Thanks-a-mundo for the article post.Really thank you! Much obliged.
Wow, great article. Fantastic.
Really appreciate you sharing this blog. Great.
I really like and appreciate your blog article.Really looking forward to read more. Cool.
Really enjoyed this article post.Thanks Again. Great.
Really appreciate you sharing this post.Really thank you! Keep writing.
คาสิโนออนไลน์นับได้ว่าเป็นวิธีที่เยี่ยมงามๆสำหรับการนักพนันเพราะเหตุว่าอีกทั้งสบายรวมถึงปลอดภัย เล่นที่ใดตอนไหนก็ได้ซึ่งพูดได้ว่าคุณสามารถทำเงินได้ตลอดระยะเวลา UFABETได้สะสมทุกเกมไว้คอยคุณแล้วแค่เพียงสมัครเข้ามาก็ทำเงินได้ในทันทีทันใด
An insightful blog post right there mate ! Thanks for the post .
I value the blog.Really thank you! Really Great.
This is one awesome article post.Really thank you! Will read on…
Thanks for the blog.Really thank you! Awesome.
Discover cost-effective and effective Server Rental in Chennai for services of all sizes.
Thanks again for the post. Great.
I value the article post.Much thanks again. Really Great.
Really informative article post.Thanks Again. Will read on…
I loved your article post.Much thanks again. Will read on…
Awesome blog article.Really thank you! Much obliged.
Major thankies for the post.Really looking forward to read more. Awesome.
I am so grateful for your post.Really looking forward to read more. Cool.
Thanks so much for the blog.Much thanks again. Will read on…
Strategy your IT tasks effectively with a distinct IT Roadmap to attain long-lasting success.
I am so grateful for your article post. Will read on…
Howdy! I could have sworn I’ve been to this blog before but after browsing through some of the post I realized it’s new to me. Nonetheless, I’m definitely happy I found it and I’ll be book-marking and checking back often!
testbericht stereophile (messungen) thought so too psb stratus mini
Finding excellent Uttarakhand food in Bangalore used to be tough, however Joshital has fixed that issue!
If you’re puzzled about government-backed programs, this guide on Student Loan Forgiveness Programs Everything You Need to Know is for you.
Fantastic article.Thanks Again. Keep writing.