
Alkylhalides (R-X, X = Cl, Br, I) (A-level chemistry)

Alkyl halides (R-X, X = Cl, Br, I)
These are compounds with at least one halogen atom attached to the parent chain.
Classification of monosubstituted alkyl halide
As seen earlier, they are classified as alcohol.
There are three classes of alkyl halide depending on the number of alkyl groups attached to a carbon atom that carry a halide.
(a) Primary alkyl halides have one alkyl group on the carbon atom that carries a halide.
Examples
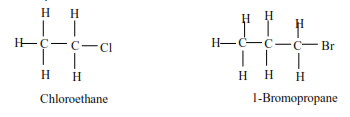
(b) Secondary alkylhalide have two alkyl groups attached to a carbon atom that carry a halide
Examples
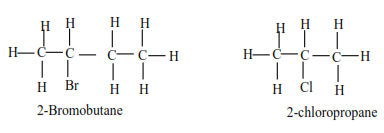
(c) Tertiary alkyl halide
Tertiary alkyl halides have three alkyl groups attached to the carbon atoms that carry OH group
Examples
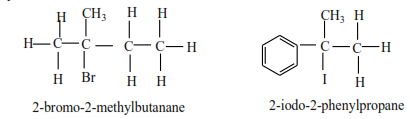
Preparation of alkyl halides
- By reacting alkenes with halogen halide
Example
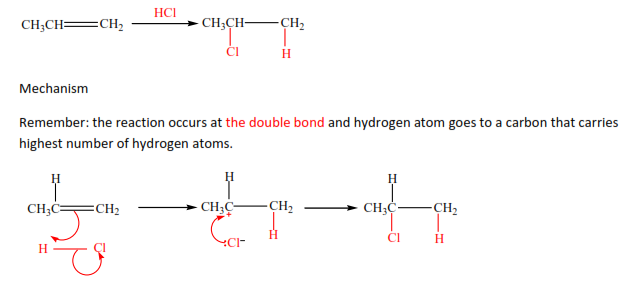
Exercise
Complete and write a mechanism for the following reactions (mark yourself after)
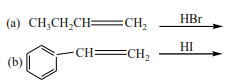
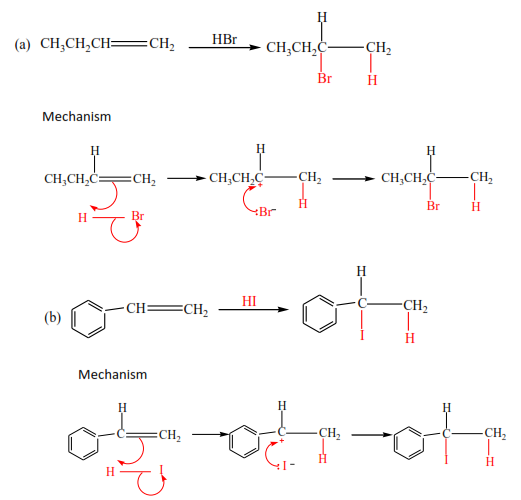
Mark yourself
In these reactions, the double bond breaks and hydrogen halide adds itself across the carbon atoms that were forming the double bond. Hydrogen atom adds to a carbon atom the carries the highest number of hydrogen atoms of those that form a double bond. Make sure you account for the positive and negative charges.
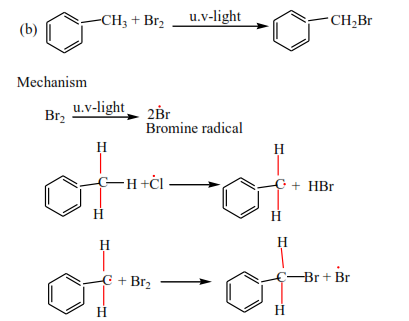
(b) By reacting chlorine gas or bromine with alkanes. The reaction occurs in the presence of u.v light. This was dealt with when dealing with reactions of alkanes.
Complete the following equation and write a mechanism
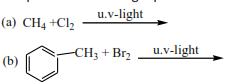
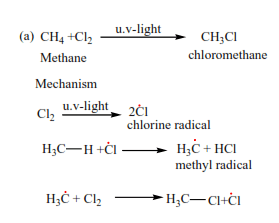
Mark yourself
These reactions follow a free radical mechanism, be keen to observe the initiation and formation of free radicals as the reaction proceeds. Every dot (un paired electron) must be accounted for.
3. By reacting alcohols with
(a) Phosphorus halide
Examples
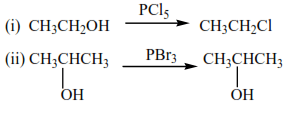
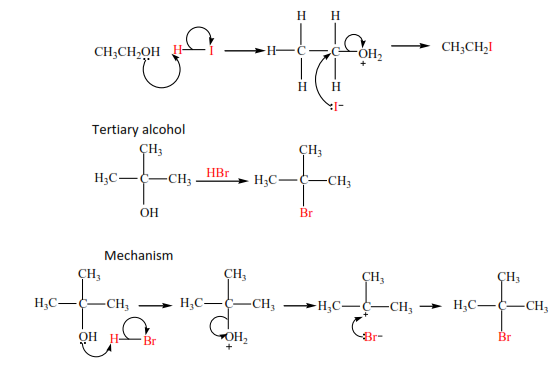
With HX (X= Cl, Br, I)
HI and HBr react readily with alcohols but the reaction of HCl is catalyzed with anhydrous zinc chloride. The mechanism of the reaction depends on the class of alcohol. Though secondary alcohols react like either primary or tertiary alcohols.
Examples
Primary alcohol

Mechanism
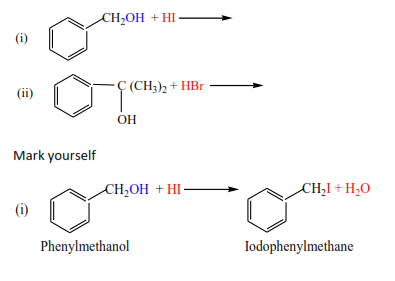
Exercise
Complete and write a mechanism, thereafter mark yourself
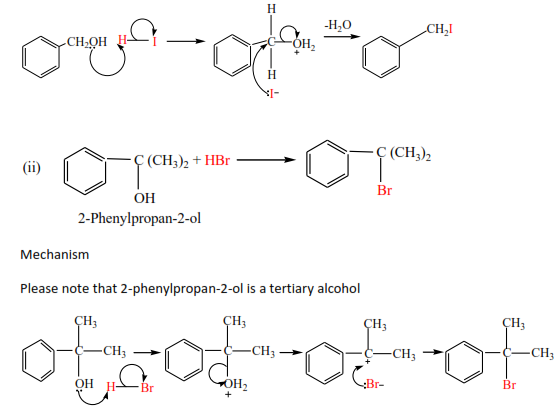
(c) The reaction of HCl with alcohol is catalyzed by anhydrous zinc chloride because HCl bond is strong. Primary alcohol does not react, secondary alcohol reacts slowly, and tertiary alcohol reacts faster.
Example
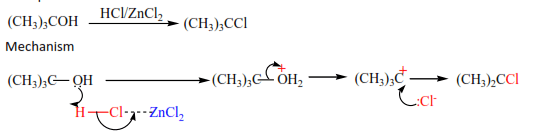
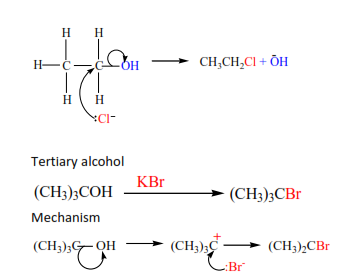
(d) The reaction of alcohol with KX (X= Cl, Br, I)
The mechanisms depend on the class of alcohols. The mechanism of secondary alcohols
Primary alcohol
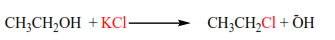
Mechanism
CHEMICAL PROPERTIES
(a) Formation of alkenes
Alkyl halides react with hot alcoholic potassium hydroxide to form alkenes. The mechanism depends on the class of alkyl halide but the mechanism of reaction for secondary alkyl halide is similar to that of the primary or tertiary alkyl halide. These reactions were also considered when we dealt with the preparation of alkenes.
(i) Example for primary alkyl halides,
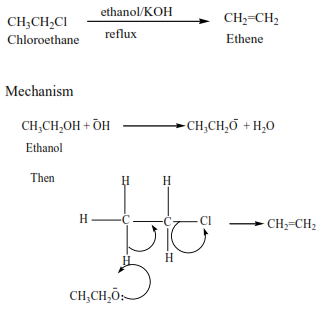
Example II
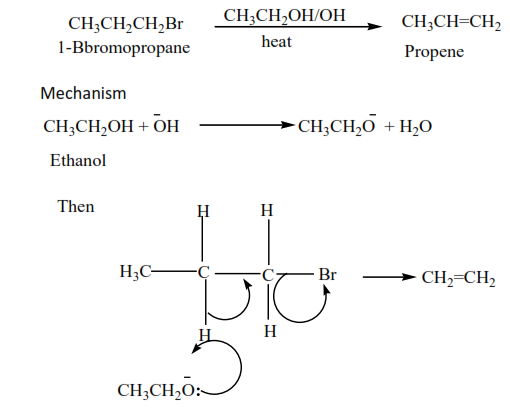
Exercise
Complete and write a mechanism
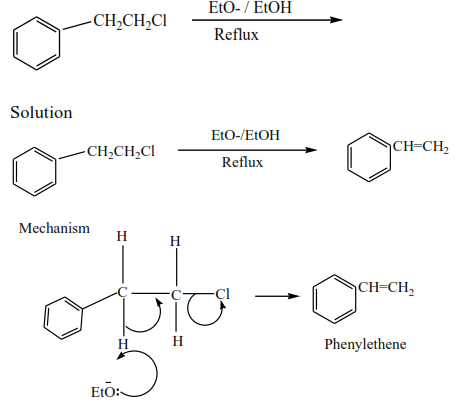
(ii) Secondary alkyl halides undergo the same mechanism as primary alkyl halides or that of tertiary alkyl halides
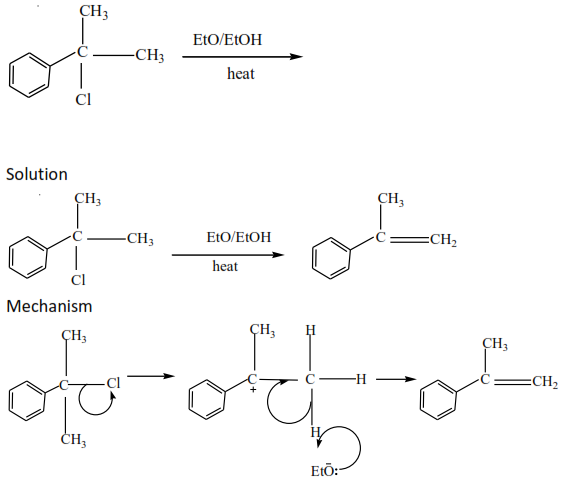
(iii) Tertiary alkyl halides undergo a mechanism called E1 or elimination unimolecular because a water molecule is eliminated and the slowest step involves one molecule only.
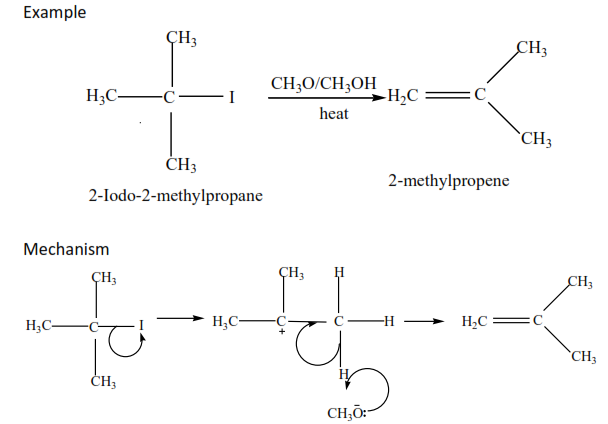
Exercise
Complete and write a mechanism
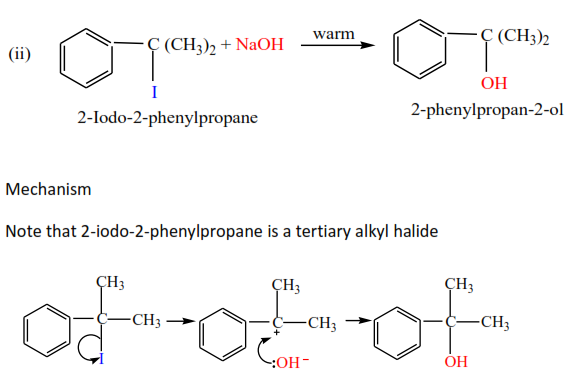
(b) Reaction of alkyl halide with alkalis (NaOH or KOH)
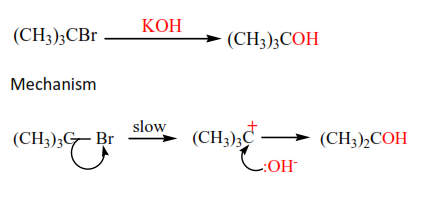
Alkyl halides react with hot alkalis to form alcohols. The secondary alkyl halides react by either mechanism of primary or tertiary alkyl halides.
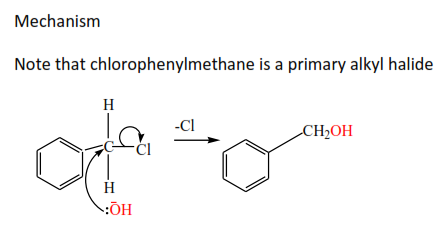
Primary alkyl halide
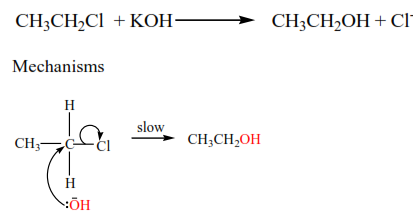
The mechanism is described as SN2 (substitution nucleophilic bimolecular)because a nucleophile (-OH) substitutes a halide atom and the slow step in the reaction involved two species i.e. –OH and alkylhalide.
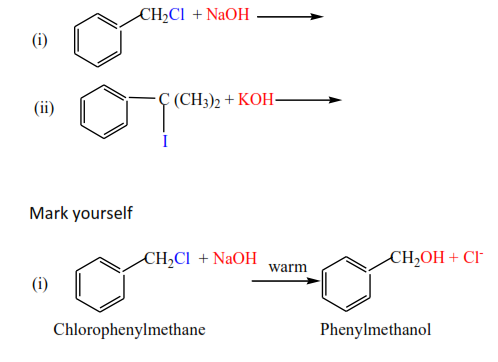
Exercise
Complete and write a mechanism, thereafter mark yourself
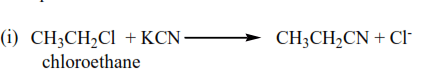
(c) Reaction with potassium cyanide
Alkyl halides react with potassium cyanide to form nitrile. This reaction is important because it increases the carbon chain by one carbon atom. Primary alkyl halide undergoes SN2 while tertiary alkyl halide undergoes SN1 mechanism whereas secondary alkyl halides undergo either mechanism.
Examples
Mechanism (note that chloroethane is a primary alkyl halide)
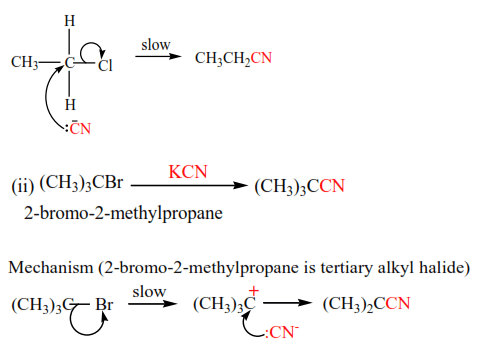
(d) Coupling reaction
Two alkyl halides couple in presence of sodium and dry ether or zinc-copper couple to form alkane of twice the number of carbon atoms as the parent alkyl halide.
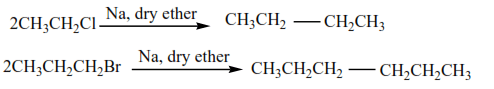
(e) Formation of Gridnard’s reagent
Alkyl halides react with magnesium in presence of dry ether to form compound called Gridnard’s reagent.
Example
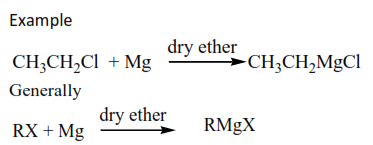
Gridnard’s reagents are important synthetic molecules that enable us to increase the parent carbon chain by unlimited number of carbon atoms in synthesis.
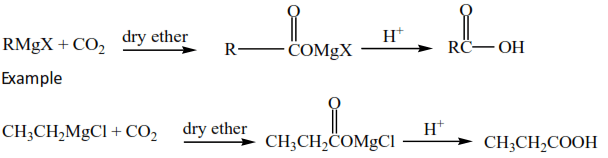
(i) Gridnard’s reagent reacts with carbon dioxide to produce carboxylic acid. The parent chain increase by one carbon atom.
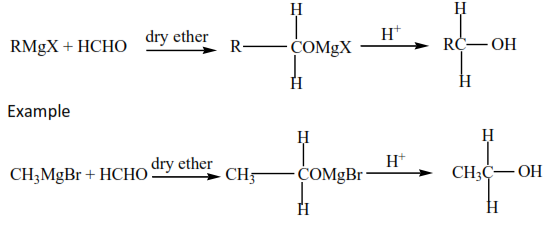
(ii) Gridnard’s reagent reacts with methanal to produce primary alcohols. The parent chain increase by one carbon atom.
(iii) Gridnard’s reagent reacts with aldehydes to produce secondary alcohols.
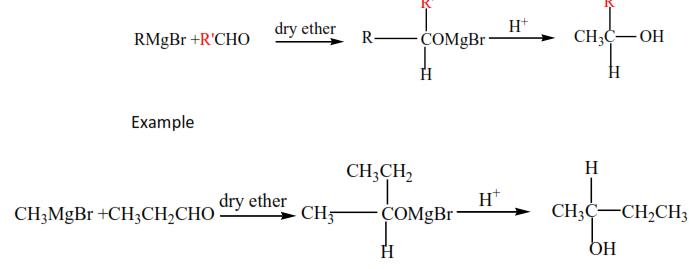
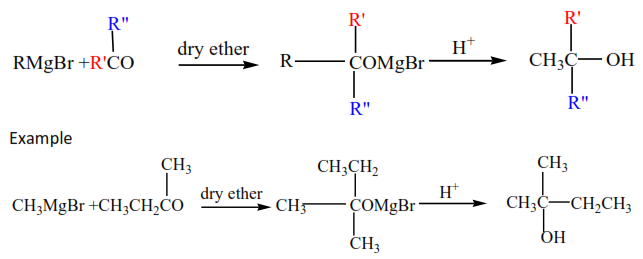
(iv) Gridnard’s reagent reacts with ketones to produce secondary alcohols.
Watch this video
Sponsored by The Science Foundation College + 256 753 80 27 09
Compiled by Dr. Bbosa Science
Thank you

Thanks for sharing your wisdom with us. Clothes & Accessories
You really have a way with words. 500 ka redeem code
Learn about the MBBS Fees Structure in Karnataka for top-ranked colleges.
Explore the latest government college cutoffs at MBBS Cutoff Of Government Medical Colleges in Delhi.
Enjoy exclusive benefits by applying the Raja Luck Invite Code.