
Amine (A-level organic chemistry)

Amines
These are organic derivatives of ammonia
Classification
According to the number of alkyl groups attached to the nitrogen atom
(a) Primary amines: have only one alkyl group attached. i.e. RNH2
Example
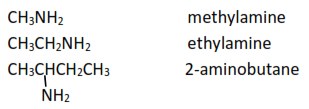
(b) Secondary amines: have two alkyl groups attached to a nitrogen atom, R2NH
Examples

(c) Terrtiary amine: have 3 alkyl groups on the nitrogen atom i.e, R3N, where R is an alkyl group.

Physical properties
Boiling and melting points
(a) Amines are polar compounds and therefore have higher melting and boiling points than non-polar compounds of similar molecular mass due to dipole-dipole intermolecular forces or hydrogen bonding,
(c) Primary and secondary amines have higher melting points and boiling points than tertiary amines because tertiary amines do not form intermolecular hydrogen bonds
(c) Primary amines have higher melting and boiling points than secondary amines because they form many hydrogen bonds per molecule.
Trial 1
Explain why the boiling points of tertiary amines are lower than those of primary amines of similar molecular masses.
Solution
Primary amines form intermolecular hydrogen bonds whereas tertiary amines do not. The hydrogen bonds require higher temperatures to break. Generally, primary amines are more soluble than secondary amine than tertiary amine.
Solubility
Amines are soluble in water because they can form hydrogen bonds with water but the solubility decrease with alkyl chain length.
Basicity of amines
Like ammonia, amines dissolve in water to form alkaline solution.
RNH2 + H2O ↔ RN+H3 + OH-(aq)
The strength of the alkaline solution is measured by the function Kb
The higher the Kb the stronger the base.

The ability to form alkaline solution, by amines, is due to the presence of a lone pair of electron on the nitrogen atom.
(i) Groups (such as alkyl groups) that donate electrons, increase the electron density of the lone pair on the nitrogen atom. This increases the ability of a lone pair of electrons to attract a proton from water and form stronger alkaline solutions.
Thus, secondary amines are stronger bases than primary amines than ammonia because secondary amine has two electron-donating groups, primary amines one, whereas, amines have none.
However, tertiary amines are weaker bases than either secondary or primary amines because their iminium ions are poorly solvated or hydrated. or the ammonium ion formed is not stable.
Groups that withdraw electrons from nitrogen atoms like phenyl group, make amines weaker bases because they reduce the ability of the lone pair of electron on the nitrogen atom and its ability to attract a proton from water.
Exercise
Explain the following observations
(i) The basic strength of the following amine is in order.
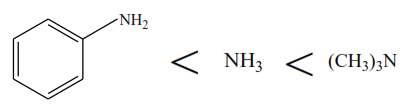
Solution
Trimethylamine is a stronger base than ammonia because the methyl groups donate electrons to the nitrogen atom. This increases the density of alone pair of electron on the nitrogen atom on trimethylamine and its ability to attract a proton from water and form hydroxyl ions.
Phenylamine is a weaker base than ammonia because the phenyl group pulls electrons from the nitrogen atom. The reduction of electron density of alone pair on the nitrogen atom reduces the ability of the lone pair to attract a proton from water and form hydroxyl group.
(ii) The boiling points of the following amines are
CH3CH2CH2NH2 = 66.80C
CH3CH2NHCH3 = 45.4
(CH3)3CN = 7.70C
Solutions
Propylamine has the highest boiling point because it has two hydrogen atoms on the nitrogen atom which form many intermocular hydrogen bonding. The hydrogen bonds necessitate high temperature to break; hence high boiling point.
Ethylmethylamine has one hydrogen bond on the nitrogen atom and thus forms fewer hydrogen bonds leading to lower boiling points.
Trimethylamines lack a hydrogen atom on a nitrogen atom and are incapable of forming intermolecular hydrogen bond leading to the lowest boiling point.
(iii) The acid constants Ka for the following amines are:
| Amine | Ka (moldm-3) |
| (CH3)3N | 9.70 |
| CH3CH2CH2NH2 | 10.67 |
Solution
Trimethylamine (CH3)3N is a weaker base than propylamine because it form unstable aqueous ammonium ions
Chemical properties
- They react with acids to form salts
Example

2. Primary and secondary amines react with alkanoyl halides to form amides
Example
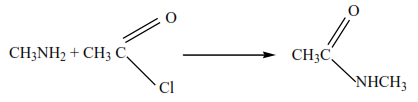
Mechanism

Exercise
Complete and write a mechanism
CH3COBr + CH3CH2NH2 →
3. Primary amines undergo condensation reaction with carbonyl compounds between pH 4 -5 to form imines. At lower pH the amine is protonated as well making it a weaker nucleophile.

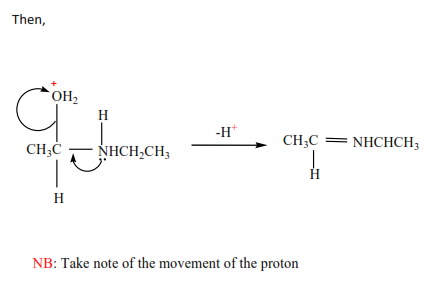
Exercise
Complete the following equations and write appropriate mechanism.

Distinguishing between primary, secondary and tertiary amines
- Reagent: nitrous acid (NaNO2, HCl <0-50C))
Observation
Primary amine: effervescence
Equation
RNH2 + NaNO2 + 2HCl → RCl + N2(g) +H2O + NaCl
- Secondary amines: yellow oil liquid
Equation
R2NH + NaNO2 + HCl → R2N-NO + NaCl + H2O
- Tertiary amines: no observable change due to the formation of soluble diazonium salts
Equation
R3N + NaNO2 + 4HCl → RN2Cl + 2RCl + NaCl + 2H2O
- Aromatic amine: no observable change due to the formation of soluble diazonium salts
Equation
ArNH2 + NaNO2 + 2HCl → ArN2Cl + NaCl + 2H2O
Reaction of aromatic diazonium salts
B. The Hinsberg test
Procedure: a mixture of small amount of the amine and benzenesuplfonyl chloride is shaken potassium hydroxide, time allowed for the reaction to take place and then the mixture is acidified.
Primary amine for a colorless solution with potassium hydroxide, forming a precipitate when the mixture is acidified.
Secondary amine: forms a precipitate with potassium hydroxide insoluble when the mixture is acidified
Tertiary amine: no observable change
PREPARATION OF AMINES
- The reaction of alkyl halides with ammonia
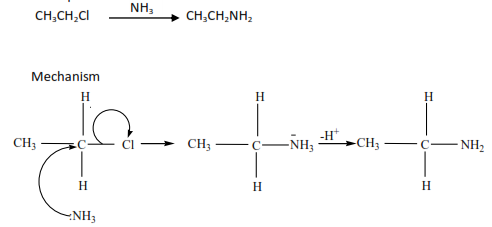
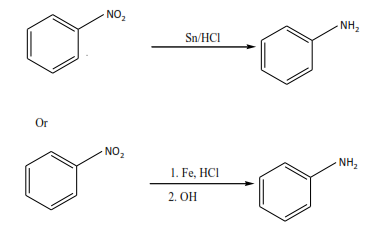
(b) By reduction of nitroalkane
3. By Hofmann degradation
Amides with no substituent on the nitrogen atom react with solution of bromine or chlorine in sodium hydroxide to yield amines.


Watch this video
Sponsored by The Science Foundation College +256 753 80 27 09
Compiled by Dr. Bbosa Science

This is awesome ! Thank you so much, Dr. Bbosa. This site is very resourceful and indeed you are a true educationist. As a teacher trainee (Chemistry and Mathematics), I am greatly inspired
You have a real knack for this topic. Computer & Accessories
I really enjoyed reading this. Well done! Tech News