
An experiment to determine the saturated vapour pressure and boiling point of water

Setup
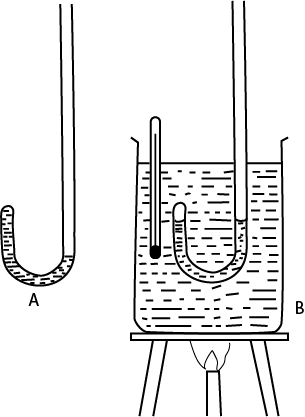
- Atmospheric pressure, H. is deterined using a mercury barometer
- Water is trapped in a J tube as shown in A
- The J-tube in and its content is transferred into a beaker of water B and a thermometer is inserted as shown in B.
- Water in the beaker is heated until it boils, and the boiling point is obtained from the constant reading of the thermometer.
- It also noted that the levels water in the closed and open the J-tubes are at the same level indicating that water boils when its saturated vapor pressure is equal to the atmospheric pressure H.
Please obtain free notes, exams and marking guides of Physics, chemistry, biology, history, economics, geography … from digitalteachers.co.ug website.
Thanks
Dr. Bbosa Science
CATEGORIES General
TAGS Dr. Bbosa Science
