
Basic trendsof amines

- Bacisity of amines
Like ammonia, amines dissolve in water to form alkaline solution.
RNH2 + H2O ↔ RN+H3 + OH-(aq)
The strength of the alkaline solution is measured by the function Kb
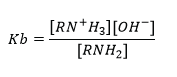
The higher the Kb the stronger the base.
The ability to form alkaline solution, by amines, is due to the presence of a lone pair of electron on the nitrogen atom.
(i) Groups (such as alkyl groups) that donate electrons increase the electron density of the lone pair on the nitrogen atom. This increases the ability of alkylamine to attract a proton from water to release hydroxide ions.
Thus, secondary amines are stronger bases than primary amines than ammonia because secondary amine has two electron donating groups, primary amines has one, whereas, amines have none.
However, tertiary amines are weaker bases than either secondary or primary amines because their iminium ions are poorly solvated or hydrated.
(ii) Groups that withdraw electrons from nitrogen atoms like phenyl group, make amines weaker bases because they reduce the ability of the lone pair of electron on the nitrogen atom and its ability to attract a proton from water.
For the same reason ethylmethylamine is a stronger base than propylamine than trimethylamine than phenyllamine
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much

You have a way with words, truly. Sports Fitness & Outdoor
I’m constantly learning from your posts. 500 ka redeem code
Access top institutions with MBBS Admission Through Management/Nri Quota in Andhra Pradesh.
Test your skills and luck with the immersive Raja Luck Game.
Comprehending Why Backlinks Matter has changed the method I approach SEO. Thanks for sharing.
Choose from a wide variety of high-performance servers with Server Rental in Chennai.