
Bonding, (A-level Inorganic chemistry)

Chemical bonding
This chapter teaches the different types and names of bonds that exist in substances that keep their constituent particles together.
We will understand how these bonds affect the physical properties of substances such as melting, boiling, solubility in different solvents, and conductivity of electricity.
In the end, we are able to explain why some substances have high and others low have boiling and melting points; why some substances conduct electricity while others do not.
A chemical bond is a force of attraction that keeps atoms, ions, or molecules together in chemical compounds or solid.
There are three types of chemical bonding namely: –
1. Ionic (electrovalent) bonding.
2. Covalent bonding.
(a) Normal covalent bonding.
(b) Dative (coordinate) bonding.
3. Metallic bonding.
4. Van der Waals forces
Elements react to attain stable (doublet or octet) electronic configurations of the noble gases.
Ionic bonding
This involves the transfer of one or more electrons from one atom to another, e.g.
Na → Na+ + e–
(1s22s22p63s1) (1s22s22p6)
Cl + e– → Cl–
(1s22s22p63s2 3p5) (1s22s22p6 3s2 3p6)
By losing one electron, sodium atom achieves a stable electronic structure (similar to that of neon) while the addition of an electron to a chlorine atom makes it achieve a stable electronic configuration (similar to that of argon).
It is the electrostatic attraction resulting from the opposite charges that constitute the ionic or electrovalent bond between Na+ and Cl– ions in sodium chloride.
Factors that favor the formation of the ionic bond
- One of the two atoms involved should have lower ionization energy and the other must have a higher electron affinity.
For instance, elements such as aluminium metal form compound most of which are not ionic due to the high ionization energy of aluminium (i.e., a large amount of energy is needed to remove all the 3 valence electrons from an aluminium atom).
2. The electronegativity difference between the two atoms involved must be high.
For example, a compound formed between an element of group 1A and an element in group 7B is more ionic than one formed with a group 2A element and group 7B element. Usually, ionic compounds are formed between elements of the left-hand side and elements of the right-hand side of the periodic table.
Characteristic properties of ionic compounds
- They consist of ions and not molecules.
- They are electrolytes, i.e., when dissolved in water or when fused, conduct an electric current followed by decomposition into constituent elements.
- They are solids of high melting points. The melting point increases with the strength of ionic character for instance, the melting point of NaCl > NaBr > NaI decrease in that order; because the strength of the ionic bond decreases in the same order due to decreasing electronegativity of the anions.
- They are insoluble in organic solvents but many of them are soluble in water.
Factors affecting the strength of ionic bond
(i) Size of the cation and anion; ionic bonds are stronger when the size of the cation is big and that of the anion is small due to low polarization, e.g. NaCl is more ionic than LiCl.
(ii) Electronegativity/electropositivity differences; the bigger the difference of these factors between the reacting elements, the stronger the ionic bond e.g. NaCl is more ionic than NaI.
Polarization of ions
In the formation of ionic molecules, when two oppositely charged ions (i.e. cations and anions) of ionic molecules come close to each other, the cations attract the electron charge cloud of the outermost shell of the anion towards itself and hence the symmetric shape of the anion gets distorted (i.e. deformed or polarized). The phenomenon in which the symmetric shape of the anion gets deformed by the approach of a cation closer to it is called polarization of the ion (figure below).
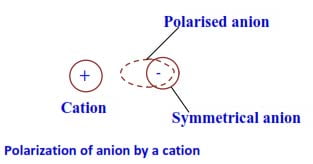
In other words, a small cation with a big charge attracts electron clouds of anion changing its shape from spherical to oval.
Polarization leads to partial sharing of electron between a cation and anions converting an ionic bond into a partial covalent bond. Ionic compound with polarized bond acquire characteristics of covalent compounds such as low boiling, melting points, and solubility in organic solvents.
The ability of a cation to polarize an anion is called its polarizing power or its polarizing ability. The tendency of an anion to get polarized by a cation is called its polarizability.
Factors affecting the magnitude of polarizing power of a cation
(a) Magnitude of positive charge on the cation:
The higher the positive charge on the cation, the more strongly it attracts the outermost shell electron cloud of the anions towards itself and hence polarizes a given anion more strongly. E.g. the polarizing power of isoelectric cationic in the order Na+< Mg2+ < Al3+. This explains why salts of aluminium are covalent and thus soluble in organic solvents and have lower melting and boiling points than those of sodium salts.
Trial 1
The table below shows the ionic radii of sodium, magnesium, and aluminium and the melting points of the chlorides formed by the three cations.
| Na+ | Mg2+ | Al3+ | |
| Ionic radii (nm) | 0.095 | 0.065 | 0.050 |
| Mpt./0C | 801 | 712 | 180(sublimes) |
(i) Calculate the charge/radius ratio for each cation. (1½ marks)
(ii) State and explain the trend in the melting points of the chlorides of the three metals. (5 marks)
(b) Size of cation:
Small cations have high polarizing power than big cations of the same charge i.e. Li+ > Na+ >K+. Small positive ions with high charge have the highest electrical fields and therefore the highest polarizing power. That is why compounds of lithium, beryllium, and aluminium are much more covalent than corresponding compounds of other elements in the periodic table groups.
(c) Electron configuration
If we compare the polarizing power of two cations which have the same positive charge on them and same or almost same size, then the cation having ns2 np6nd1-10 as its valence-shell configuration will polarize the given anion more strongly than the cation having ns2np6 as its valence shell configuration because d-electrons shield the nucleus poorly compared to s– and p-electrons. For example, Cu+ and Na+ ions have almost the same size (Cu+ = 0.096 nm, Na+ = 0.096 nm) and the same charge (=+1), but the Cu+ ion polarizes Cl– ion in CuCl more strongly than Na+ in NaCl. This is due to the fact that Cu+ ion has 3s23p63d10 configuration while the sodium ion has 2s22p6 configuration (8electrons). The 3d10 electrons in Cu+ shield the nucleus less effectively than the 2s2 and 2p6 electrons in Na+ ion do.
Factors affecting the polarizability
- The magnitude of the negative charge on the anion. The higher the negative charge on the anion, the more easily and strongly its outer-most electron cloud can be attracted by the cation.
- Size of the anion. The larger the size of the anion, the higher its polarizability will be; that is the reason why fluorides are more ionic than other halides and oxides are more ionic than sulphides.
Pajan’s rules of covalency
These are conditions that when possessed by cations and anions of a given molecule, make the ionic bond partially or fully covalent.
- The cation should have high positive charge on it.
- The cation should be small in size.
- The cation should have ns2np6nd10 configuration which has high polarizing power.
- The anion should have a high negative charge.
- The anion should be large in size.
Trial 2
(a) Explain what is meant by the term melting point. (1mark)
(b) State the factors which affect the melting points of
(i)Metals.
(ii) Nonmetals.
(c) Explain the trend in melting points of the elements in group (II) and group (VII) of the periodic table. (8 marks)
(d) Explain why the transition metals of period 4 tend to have higher melting points than non-transition metals of the same period. (2 marks)
(e) The table below shows the melting points of some compounds.
| Compound | melting point / K |
| Aluminium oxide Aluminium chloride Calcium oxide Calcium chloride |
2290 451 2850 1051 |
Explain why:
(i) The melting point of aluminium chloride is abnormally low compared to calcium chloride. (2 marks)
(ii) The melting point of calcium oxide is much higher than that of calcium chloride. (2 marks)
Covalent bonding
This is divided into two types, namely, the normal covalent bond and the dative or a coordinate bond.
(a) The normal covalent bonding
This involves sharing of one or more pairs of electrons to attain stable electron configurations similar to those of noble gases. Considering a chlorine atom, which has seven electrons in its outermost shell, if one electron is provided by each atom and shared equally, then each chlorine atom can acquire a complete octet configuration as it is called (fig. 3.2) thereby forming a chlorine molecule. The shared pair of electrons constitutes a covalent bond.

Fig.2 Formation of a covalent bond by chlorine atoms
Factors that favor the formation of covalent bonds
1. The elements involved must have high ionization energy or low electron affinity.
E.g. the ionization energy of hydrogen is quite high, the electron affinity is low, and therefore the formation of either H+ or H– is not favored.
2. The electronegativity differences between the elements involved must be very small.
Characteristics of covalent compounds
a) They consist of discrete molecules.
b) They are non-electrolytes.
c) They have low boiling and melting points.
d) They are insoluble in water and other polar solvents but soluble in organic or nonpolar solvents.
(b) Dative or coordinate bonding
The dative bond is like a covalent bond once formed except that both electrons in the shared pair are provided by one atom. The atom providing the two electrons is called the donor and the atom which accepts the two electrons is the acceptor. The donor atom must have an unshared pair of electrons available and such a pair of electrons is called a lone pair. An example is reaction of ammonia with H+ or AlCl3. By reacting with ammonia, H+ attains doublet structure of helium whereas Al3+ attains an octet configuration of argon (fig. 3 and 4 respectively).

Fig. 3 Formation of dative bonds between ammonia and hydrogen ion

Bond polarity
Consider the molecule HF. As fluorine is more electronegative than hydrogen, the fluorine atom pulls the shared electron pair towards itself, i.e. Hd+→Fd–.
Such a molecule is said to be polar and the bond is said to be intermediate type, i.e., partly covalent and partly ionic. The larger the electronegativity difference between the atoms involved in the bond, the greater the polarity. The attraction or repulsion of bonding electrons by an atom or a group of atoms is called the inductive effect. A dipole moment is the measure of the degree of polarity of the bond.
When two and opposite charges +e and -e are at a distance, l, the system is called a dipole and is characterized by a dipole moment (μ), which is the product of the charge, e, and the distance, l, between the charges, i.e. μ = e l.
The applications of the dipole moment
(i) The dipole moment helps to predict whether a molecule is polar or non-polar. As µ = q X d, greater is the magnitude of dipole moment, higher will be the polarity of the bond. For non-polar molecules, the dipole moment is zero
(ii) The percentage of ionic character can be calculated as
Percentage of ionic character = µobserved/ µionic x 100
(iii) In the determination of symmetry: Symmetrical molecules have zero dipole moment although they have two or more polar bonds.
(iv) It helps to distinguish between cis- and trans-isomers. Usually, cis-isomer has higher dipole moment than trans- isomer.
(v) It helps to distinguish between ortho, meta, and para-isomers. The dipole moment of para- isomer is zero. The dipole moment of ortho- isomer is greater than that of meta-isomer.
Significance of bond polarity
(i) Polar compounds are soluble in water and other polar solvents whereas nonpolar are not.
(ii) Polar compound have higher boiling and melting points than nonpolar compounds of similar molecular mass
The dipole is indicated by using an arrow whose head points to the negative end and the tail at the positive end of the molecules, i.e. H→F. If two dipoles come together head to tail H→F; there is a net attraction between the hydrogen and fluorine atoms that are closest together. i.e.
H→F ………………. H→F
However, a molecule made up of more than two atoms and having polar bonds may or may not have a dipole moment.
This depends on whether the molecule is symmetrical or not. For the symmetrical molecules, the individual dipole moments of the polar bonds in the entire molecule cancel, e.g., the unsymmetrical trichloromethane (CHCl3) has a dipole moment whereas as tetrachloromethane (CCl4), a symmetrical molecule has none (fig. 5).

Fig. 5 The bonding in CHCl3 and CCl4
Therefore, the lack of a dipole moment where it might be expected is an indication of symmetry within a molecule.
Trial 3
The dipole moments of BeCl2, CO2, BF4, CCl4 are zero while those of H2S, SO2, CHCl3 are not. Explain
Trial 4
Explain the following observation: CCl4 is non-polar while CHCl3 is polar.
Trial 5
Explain why carbon tetrachloride molecules are not polar yet the bonds in carbon tetrachloride are polar. (03marks)
Hydrogen bonding
A hydrogen bond is a dipole-dipole attraction that occurs between a hydrogen atom attached to a strongly electronegative atom and a second strong electronegative atom with a lone pair of electrons.

Fig. 6 Hydrogen bonds in a solution of HF in water.
The hydrogen bonds in HF are so strong that, they persist even in the vapor state and gaseous hydrogen fluoride consists mainly of a mixture of H2F2 and H3F3 molecules. The hydrogen bonding of aqueous HF is shown below (fig. 6).
Effects of hydrogen bonding
1. Compounds with intermolecular hydrogen bonds have high boiling and melting points.
2. Compounds that form hydrogen bonds with water are soluble in water.
3. Compounds that form hydrogen bonds are viscous.
These effects increase with the strength of hydrogen bonds and the strength of hydrogen bonds increases as the electronegativity of the central atom increases.
Trial 6
Explain the following observation:
(i) The boiling point of water is 1000C while that of H2S is -60.750C.
Trial 7
The table below shows the boiling points of the hydrides of some elements in a group of the periodic table

Explain:
(i) The abnormally high boiling point of NH3.
(ii) The general trend in the boiling points of PH3, ASH3, and SbH3
Metallic bonds
‘Metallic bond’ is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions.
In the metallic bond; each metal atom releases its valence electrons in a crystal pool. It is these `free’ electrons that `cement’ the positive ions together (fig. 7). The strength of metallic bonds increases with the number of electrons contributed by the metal atoms to form metallic bonds. This explains why the melting points of group II metals are higher than the corresponding group I metals.

Fig. 7 Cross-section of metallic crystal.
Factors that favor the formation of metallic bond
1. Low ionization energy.
2. Low electronegativity.
Properties of metallic substances
1. Conductors of heat and electricity due to the presence of free electrons which can move through the metal structure when it is connected to a source of electricity. The electrons are very small compared to the gaps between positive ions.
2. They are malleable and ductile because metal atoms slip over one another easily when subjected to stress or strain.
The factors that affect the strength of a metallic bond include:
(i) Total number of delocalized electron; the more the stronger the bond.
For instance, transition elements use s- and d-orbital electrons to form strong metallic bonds the reason why they have very high melting and boiling points. Another comparison commonly used is that of group II elements that use two electrons in formation of the metallic bond and have higher boiling points than those of group I elements that use one electron in their metallic bond.
(ii) The magnitude of positive charge held by the metal cation. The bigger the charge on cation the stronger the metallic bonds due to strong attraction to electron cloud.
(iii) Ionic radius of the cation; the SMALLER the ion, the stronger the bond.
Big cations form weaker metallic bonds than small atoms because their bonding electrons are farther from the nucleus and therefore experience weaker effective nuclear charge. This explains why the melting points of group I and II elements decrease down each group.
Molecular or van der Waals bond
These are intermolecular forces that occur between nonpolar molecules as a result of momentarily induced dipoles in neighboring molecules. These are a weaker type of bonding whose magnitude increases with the size or mass of the molecules. The melting points of hydrides of group 5 elements from phosphorus and the boiling points of halogens from chlorine to iodine all increase down the group due to the increase in molecular mass.
Van der Waals forces are weak forces the reason why substances bound by these forces like iodine have low melting and boiling points. In fact, iodine sublimes at room temperature.
Trial 8
Explain the following observations:
(a) The boiling point of beryllium chloride is 4870C and that of magnesium chloride is 14180C.
(b) The polarizing power of group (II) elements are much higher than those of group I elements
(c) The elements of group II of the periodic table are harder and have higher melting points than those of group I elements.
Suggested answers
Trial 1
- Na+ = 10.5, Mg2+ = 30.8, Al3+ = 60.0
- The melting points are in order AlCl3< MgCl2< NaCl because the Al3+ has the highest charge density and polarizing power that it polarizes the Cl– form a covalent compound. The low charge density and polarizing power of Na+ make NaCl a strong ionic compound with a high melting point
Trial 2
(a) It is a fixed temperature at which a solid turns into a liquid state at a given temperature when the two states are in equilibrium.
(b)(i) Metals (number of electrons its atoms contribute to form metallic bond and size of its atoms).
(ii)Nonmetals (size of the molecules/molecular mass and shape of the molecules).
(c) (i) The melting points of group II elements decrease down the group due to the decrease in the strength of metallic bonds. i.e. the strength of metallic bonds decreases as the bonding electrons become far away from the nucleus or as the atomic radii increase.
(ii) The melting points of the elements of group VIII increase down the group due to the increase in molecular mass and thus the strength of van der Waals forces.
(d) Transition elements have higher melting points because they contribute very many electrons (from s and d-orbital) to the formation of metallic bonds and they also have small metallic radii.
(e)(i) AlCl3 has greater covalent character compared to calcium chloride because of the high charge density on Al3+ polarizes the chloride ions more strongly than Ca2+.
(ii) Calcium oxide forms stronger ionic bonds than calcium chloride due to the small highly charged oxide ion as compared to the chloride ion. Cl– ions are more polarized than the oxide ions.
Trial 3
BeCl2, CO2, BF4 CCl4 have symmetrical shapes thus the dipole moments in their molecules cancel whereas H2S, SO2,CHCl3 are angular molecules and nonsymmetrical thus the dipole moments in their molecules do not cancel to zero.
Trial 4
CCl4 is a symmetrical molecule, the dipole moments in its polar bonds cancel whereas the dipole moments in a nonsymmetrical CHCl3 do not cancel.

Trial 5
CCl4 being a symmetrical molecule, the dipole moments its polar bonds cancel
Trial 6
Oxygen atom is more electronegative than that of sulphur atom, consequently, O-H bond is more polar than the S-H bond; the hydrogen bond in water are thus stronger than those in hydrogen sulphide giving a higher boiling point.
Trial 7
(i) The boiling point of ammonia is abnormally high because it forms strong intermolecular hydrogen bonds. This is because the nitrogen atom has high electronegativity leading to polar N-H bonds.
(ii) The boiling points of PH3, ASH3, and SbH3 increase in that order due to the increase in the relative molecular masses of the molecules which increases their intermolecular forces.
Trial 8
(a) Be2+ has a higher charge density than Mg2+ due to its small size. Be2+ s polarizes Cl– more strongly than Mg2+. This makes BeCl2 covalent with low boiling point.
(b) Group II elements have bigger charges and small sizes giving them higher polarizing power.
(c) Group II elements contribute two electrons per atom to the formation of stronger metallic bonds than group I elements that contribute 1 electron per electron.
Download PDF
Watch this
Sponsored by The Science Foundation College + 256 753 80 27 09
Compiled by Dr. Bbosa Science
Thank U

So understandable notes thanks
I Think you were called by God to help others_favourable notes
Thanks for being such a guide. Electronics
Fantastic job on this article. Keep it up! Transfer Latest
Ensure a smooth admission process with MBBS Direct Admission in Gujarat.
does augmentin make you tired After eight weeks, we saw a 20 point improvement in fatigue in patients with cancer measured on a 100 point, standardized fatigue scale, Barton says
Learn about guaranteed enrollment through MBBS Direct Admission in Tamil Nadu.
Win exciting rewards and enjoy seamless gaming on Raja Luck.
Discover the excitement of Goa Game, a platform that brings traditional color prediction games to your fingertips.
Enjoy VIP advantages and concern benefits with the Diu Win Invite Code.
Satisfy your growing storage and computing needs with customized Server Rental in Pune.
Implement structured Technical Roadmap Planning to improve item advancement and IT performance.