
Carboxylic acid or alkanoic acids (A-level organic chemistry)

Carboxylic acid s
s
The carboxylic group is a combination of a carbonyl group and a hydroxyl group(OH): the name carboxylic group.

Nomenclature
- Their names end with suffic “oic” followed by the word acid.
- The carboxylic group must always be at the end of the chain and therefore it’s position need not to be specified. Take longest chain that contain the carboxylic group and start numbering from the carboxylic carbon
| Examples | Systematic name | Common name |
| HCOOH | Mechanoic acid | Formic acid |
| CH3COOH | Ethanoic | Acetic acid |
| CH3CH2COOH | Propanoic acid | Propionic acid |
| CH2CH2CH2COOH | Butanoic acid | Butyric acid |
| CH3CH(CH3)COOH | 2-methylpropanoic acid | Isobutyric acid |
Physical properties
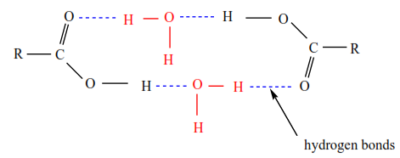
(1) Lower members are liquids and higher members are waxy solids. With boiling points which are higher than expected, this because, their molecules are associated with hydrogen bonds.
(2) Lower members are soluble in water but the solubility decreases with the increasing molecular mass of carboxylic acid. This is because carboxylic acids are capable of the formation of hydrogen bonds with water.

(3) Melting points
| Compounds | Mpt (0C) | Bpt (0C) | Ka |
| HCOOH | 8 | 100.5 | 1.7 x 10-4 |
| CH3COOH | 16.6 | 118 | 1.77 x 10-5 |
| CH3CH2COOH | 21 | 141 | 1.34 x 10-5 |
| CH3(CH2)2COOH | -6 | 164 | 1.54 x 10-5 |
| CH3(CH2)3COOH | -34 | 187 | 1.52 x 10-5 |
| CH3(CH2)4COOH | -3 | 205 | |
| CH3(CH2)5COOH | 16 | 259 |
Generally the melting point increase with the molecular mass. However, the melting points of lower carboxylic acids are relatively higher than those of higher carboxylic acids because they form stronger hydrogen bonds.
4. The acidity of carboxylic acids decreases with the increase in molecular mass due to the positive inductive effect of alkyl groups. Electron-withdrawing groups make carboxylic acid more acidic.
For example, CCl3COOH is more acidic than CH3COOH.
Methods of preparation
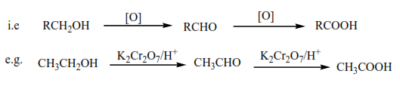
- By oxidation of primary alcohols using K2Cr2O7/H+, Na2Cr2O7/H+, or KMnO4/H+

2. Oxidation of aldehyde using K2Cr2O7/H+, Na2Cr2O7/H+, or KMnO4/H+

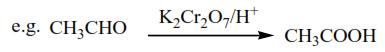
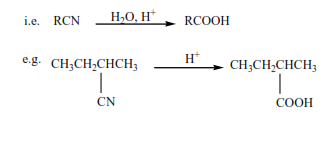
3. Hydrolysis of nitrile in presence of a mineral acid

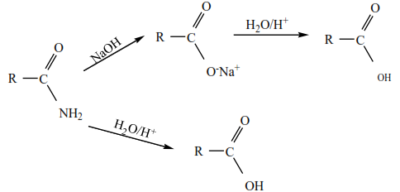
4. Hydrolysis of acid amide with a mineral acid or alkali

5. Reaction of carbon dioxide with a grignard reagent followed with hydrolysis.
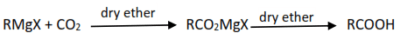

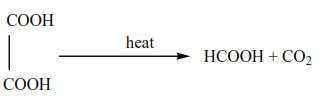
6. Preparation of methanoic acid
By heating a solution of ethane dioic acid in propane-1,2,3-triol
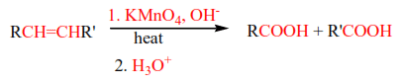
7. Oxidation of alkenes using hot alkaline KMnO4.

8. Oxidation of methyl ketone
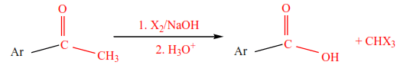

Chemical properties
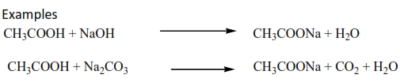
- Carboxylic acid react with base to form salts and even liberate caarbon dioxide from carbonates.
Examples

2. Reduction of carboxylic acid
Carboxylic acids are resistant to reduction by mild reducing agents. However, can be reduced through aldehydes to primary alcohols by LiAlH4 in presence of dry ether.
Example


3. Decarboxylation
This is a reaction in which a molecule of CO2 is removed from carboxylic acids.
(i) Simple carboxylic acids are not easily decarboxylated but their salts are easily decarboxylated in presence of soda lime.
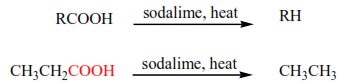

(ii) Acids with electron-withdrawing groups on α-carbon are easily decarboxylated on heating.
Example


(iii) Esterification: Carboxylic acids react with alcohol in presence of mineral acids to form es
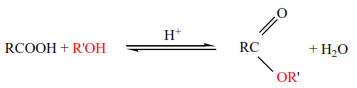
Easter have got sweet smell; this is why this reaction is used in identification of carboxylic acids
Reactivity of alcohols is in order 10 ›20 ›30, and the reactivity of carboxylic acids is in order HCOOH› CH3COOH › RCH2COOH › R2CHCOOH › R3CCOOH, due to steric hindrance. i.e. the presence of bulky groups near the site of reaction, whether in the alcohol or in acid slows esterification.
5. Formation of acid halides
Carboxylic acids (except methanoic acid) react with phosphorus halides to form acid halides
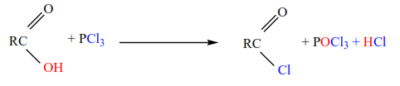


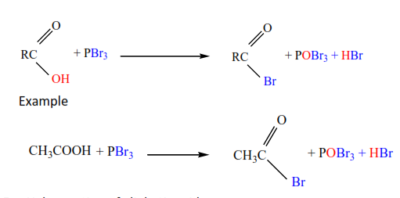
5. Halogenation of aliphatic acids
(a) Carboxylic acids (except methanoic acid) reacts with chlorine in presence of sunlight or u.v light, thereby a chlorine atom replacing an α-hydrogen.

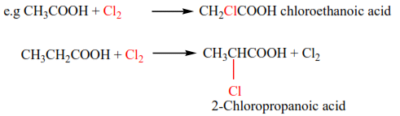
(b) Bromine replaces an α-hydrogen in presence of red phosphorous (hell-vohlard zelensky reaction)

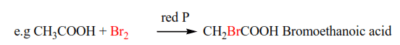
Watch this video
Sponsored by The Science Foundation college +256 753 802709
Compiled by Dr. Bbosa Science
END

Thanks for shedding light on this. Bags Wallet & Luggage
Your blog is a source of inspiration for me. 500 ka redeem code
Top MBBS Colleges in Haryana focus on academic excellence and clinical exposure.
Access exclusive features and win exciting prizes with the Raja Luck App.