
Chemical equilibrium, Kc and Kp, factors affecting chemicals equilibrium (A-level physical chemistry)

Chemical equilibrium
It is a branch of physical chemistry that deals with reversible reactions or reactions that proceed in either direction.
For example, when steam passed over heated iron, hydrogen, and iron (II)diiron (III) oxide is formed. If, however, hydrogen is passed overheated iron (II) diiron (III) oxide, steam and iron are produced. Such reactions are said to be reversible and the equation for this reversible reaction is expressed as follows:
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2 (g)
The direction of the reaction depends on the conditions: if hydrogen gas is removed as fast as it has formed, the reaction proceeds from left to right. Alternatively, if steam is removed as fast as it is formed the reaction will proceed from right to left.
By having steam and iron in a closed vessel, a state is reached at which all the four substance exist in equilibrium but the equilibrium in a balanced reaction is a dynamic one, that is, the substances are still reacting together except that the velocities of the forward and backward reaction are equal.
The equilibrium constant, Kc
The state of equilibrium of a reversible reaction at a given temperature is defined by the equilibrium constant as follows
For a reaction
3Fe(s) + 4H2O(g) ↔ Fe3O4(s) + 4H2 (g)
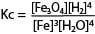
Where [ ] means concentration of the item in moles per litre or moles per dm3
For a general equation
aA + bB ↔ cC + dD
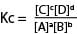
Units of Kc
The units of are derived by principles of dimension
for instance

Trail 1
Write expressions for Kc for the following reaction and state their units
- N2+ 3H2 ↔ 2NH3
- 2NH3 ↔ N2+ 3H2
- N2 + O2 ↔ 2NO
- C2H5OH + HCl ↔ C2H5Cl + H2O
Implication of Kc
When Kc is high, it implies that there are more products at equilibrium than the reactants
Application of equilibrium constant, Kc
To calculate the amounts of the reactants and product at equilibrium
Summary of characteristics of equilibrium
- Occurs at constant temperature
- Occurs in closed system
- It is dynamic equilibrium
Example 1
Hydrogen reacts with iodine according to the following equation
H2(g) + I2(g) → 2HI(g)
A mixture of 0.8 moles of hydrogen and 0.6 moles of iodine was allowed to react in a sealed tube at 4500C at equilibrium 0.2 moles of iodine had reacted.
(a) Write the expression for the equilibrium constant, Kc, for the reaction

(b)Calculate the value of (2 marks)
First we calculate the moles at equilibrium
mole of I2 at equilibrium = 0.6 – 0.2 = 0.4 moles
Moles of H2 at equilibrium
Mole of H2 that reacted = moles I2 that reacted = 0.2moles
Thus, mole of H2 at equilibrium = 0.8 – 0.2 = 0.6 moles
Moles of HI at equilibrium
1 mole of I2 that reacted produces 2moles of HI
0.2 moles of I2 produced 0.4 mole of HI
Secondly, we substitute in Kc expression

Example 2
When 8.28 g of ethanol were heated with 60 g of ethanoic acid, 49.74 g of the acid remained at equilibrium. Calculate
What mass of ethylethanoate should be present in equilibrium mixture firmed from 13.8 g of ethanol and 12 g of ethanoic acid
Ethanol and ethanoic acid react as follow:
CH3CH2OH + CH3COOH ↔ CH3COOCH2CH3 + H2O
ethanol ethanoic acid ethylethanoate water
(H= 1, C = 12, O = 16)
Solution
Relative molecular mass of ethanol, CH3CH2OH = 46

From the reaction equation
| Moles of ethanoic acid that reacted | = | Moles of ethanol that reacted | = | Moles of ethylethanoate that formed |
Mole of ethanioic reacted = 1-0.829 = 0.171moles
Moles of water formed = moles of ethanoic acid reacted = 0.171moles
moles of ethanol that remained = 0.18- 0.171
= 0.009moles


Trial 2
When 6.22moles of hydrogen were heated with 5.71moles of iodine in a sealed tube at 3560C it was found that 9.60moles of hydrogen iodide were present at equilibrium. Calculate
(i) the equilibrium constant
(ii) the moles of hydrogen iodide in equilibrium mixture formed by heating 6.41 moles of hydrogen and 10.40 moles of iodine at 3560 C
Trial 3
Nitrogen reacts with hydrogen according to the following equation
N2(g) + 3H2 (g) ↔ 2NH3(g)
- Write expression for equilibrium constant, Kc
- Stoichiometric amounts of nitrogen and hydrogen were reacted in a 2litre vessel. At equilibrium, 0.8moles of ammonia was formed
Calculate
(i) the amount of hydrogen at equilibrium
(ii) the value of equilibrium constant,.
Trial 4
Hydrogen iodide decomposes when heated according to equation
2HI(g) ↔ H2(g) + I2 (g) ∆H +11.3kJmol-1
(a) Write an expression for equilibrium constant, for the reaction (1mark)
(b) 1.54g of HI was heated in a 600cm3 bulb at 5300C. When equilibrium was attained, the bulb was rapidly cooled to room temperature and broken down under potassium iodide solution. The iodine formed from decomposition required 67.0cm3 of 0.1M sodium thiosulphate solution.
Calculate
(i) the number of moles of HI in 1.54g (1mark)
(ii) number of moles of iodine formed when hydrogen iodide decomposed (3marks)
(iii) The value of Kc
(c) State what would be the effect on if
(i) the temperature was raised from 5300C to 8000C
(ii) the volume of the bulb was increased to 1200cm3.
Trial 5
1mole of H2 and 1mole of I2 were introduced into 2litre vessel at temperature at which the equilibrium constant is 55.3. How many moles of H2, I2 and HI are there after equilibrium has been established.
Trial 6
A reaction occurs according to the following stoichiometric equation:
A + 2B ↔ 2C;
3 moles of A and 5 moles of B were brought together in a volume of 3 litres and after equilibrium has been established, 1 mole of A remained. Calculate the equilibrium constant, Kc. for the reaction
Trial 7
A reaction occurs according to the following stoichiometric equation:
2A + B ↔ 3C
and has equilibrium constant of 1/10. How many moles of B must be brought into contact with 5 moles of A to produce at equilibrium 5 moles of C.?
Trial 8
5moles of ethanol, 6 moles of ethanoic acid, 6moles of ethylethanoate and 4moles of water were mixed together in a stoppered bottle at 150C. After equilibrium had been attained the bottle was found to contain only 4 moles of ethanoic acid.
(a) (i) Write an equation for the reaction between ethanol and ethanoic acid to form ethylethanoate and water.
(ii) Suggest a mechanism for the reaction
(b) Write an expression for the equilibrium constant, Kc, for this reaction
(c) How many moles of ethanol, ethanoic acid, ethylethanoate and water are present in the equilibrium mixture?
(d) What is the value for for this reaction
(e) Suppose 1 mole of ethanol,1 mole of ethanoic acid,3 moles of ethylethanoate and 3 mole of water are mixed together in a stoppered flask at 150 C. How many moles of
(i) ethanol,
(ii) ethanoic acid
(iii) ethylethanoate
(iv) water are present
Equilibrium Constant in terms of, Kp
Watch this
When at least one of the substances in equilibrium is gases, the state of equilibrium may be defined by equilibrium constant as follows
For a reaction;
aA(g) + bB(g) ↔ cC(g) + dD(g)

Application of Kp
Like Kc, Kp, is used to predict the proportion of the products and reactants at the equilibrium point in the mixture
Example 3
AT 7000C and total pressure of one atmosphere the partial pressure at equilibrium for sulphur dioxide and oxygen are 0.27 and 0.41 atmospheres respectively. Sulphur dioxide is oxidized to sulphur trioxide according to the following equation
2SO2(g) + O2(g) ↔ 2SO3 (g)
Calculate the equilibrium constant, Kp , for the reaction (4 marks)
Solution
Partial pressure of SO3 = (1-(0.27 + 0.41)
= 0.32atm.
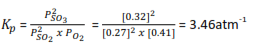
Trial 9
1mole of hydrogen iodide at 250C was introduced into a container of 20 liters
(a) Calculate the pressure of the gas, assuming ideal behavior (1mole of a gas occupies 22.4dm3 under standard conditions).
(b) The sample of hydrogen iodide considered above was raised to a temperature of 3000C and partially decomposed into hydrogen and iodine gases; at equilibrium 0.1 mole of iodine was found to be present
(i) Write an equation for decomposition
(ii) Calculate the pressure of the equilibrium mixture at 3000C, assuming no volume change.
(iii) Calculate the equilibrium constant at 3000C
(c) ∆H0f HI = 26.5kJmol-1. Explain giving reasons how you would expect the equilibrium constant to change with temperature.
Trial 10
Nitrogen reacts with hydrogen according to the following equation
N2 (g) + 3H2(g) ↔ 2NH3(g)
(a) Write the equation for the equilibrium constant for the reaction in terms of partial pressure and indicate units (2 marks)
(b) Stoichiometric amounts of nitrogen and hydrogen were reacted at 50 atmospheres and at equilibrium, 0.8 moles of ammonia were formed.
calculate
(i) the amount of hydrogen at equilibrium (2½ marks)
(ii) The value of equilibrium constant for the reaction (4 ½ marks)
Trial 11
Consider the following reaction
H2 (g) + I2(g) ↔ 2HI (g)
At a certain temperature, analysis of an equilibrium mixture of the gases yielded the following results
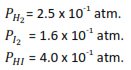
(a) Calculate the equilibrium constant for the reaction and state its units.
(b) In second experiment at the same temperature, iodine and hydrogen were mixed together with each gas at a partial pressure of 3 x 10-1 atm. what are the partial pressures of hydrogen, iodine and HI at equilibrium?
(c) In the third experiment at the same temperature, pure hydrogen iodide was injected into the flask at a pressure of 6 x 10-1 atm. What are the partial pressures of hydrogen, iodine and hydrogen iodide at equilibrium?
(d) What effect, if any, will decreasing the temperature have on the value of ? Explain your answer.
Trial 12
0.196g nitrogen and 0.146g hydrogen were heated together until equilibrium is established at a given temperature. The pressure found at equilibrium was found to be 90% its value at the beginning. Calculate the percentage composition by volume of the resulting mixture (H = 1, N = 14)
Trial 13
(a)(i) Nitrogen (II) oxide combines with O2 at 800C and 200atmosphere to form Nitrogen (IV) oxide according to equation
2NO (g) + O2(g) ↔ 2NO2(g), ∆H –xkJmol-1
(i) Write an expression for the equilibrium constant, Kp, for the reaction
(ii) Calculate, if the mixture contained 67% nitrogen (IV) oxide (3 ½ marks)
(b) State how the value of Kp will be affected if
(i) Temperature is increased
(ii) Catalyst is added
(c) The kinetic data for the reaction in (a) is shown in the table below
| Initial rate/ Nm-2s-1 | 6.8 | 27.2 | 61.2 | 108 |
| P2NO/N2M4 | 0.04 | 0.16 | 0.36 | 0.64 |
PNO =the partial pressure of NO
(i) Plot a graph of initial rate against P2NO (03mark)
(ii) Using a graph, determine the order of reaction with respect to nitrogen II oxide
(iii) Give a reason for your answer
(d) When the partial of O2 was doubled to a new constant value, the gradient of the graph in C(i) doubles
(i) Determine the order of reaction with respect to O2. Explain your answer.
(ii) Write the rate equation for the reaction in (d)
(iii) Calculate the rate constant when the initial rate is 170Nm-2s-1, PNO = 0.1NM-2, PO2 = 1.36Nm-2
(e) State the effect of the following on the rate of the reaction
(i) Having partial of nitrogen II oxide while that of oxygen is kept constant
(ii) Doubling partial pressure of both nitrogen II oxide and O2.
Relationship between and Kc and Kp
We have already seen that equilibrium constants are normally expressed in term of concentration using the symbol and that, for reaction involving gases; it is usually convenient to express the amount of gases in terms of partial pressure rather than its molar concentration using the Ideal equation
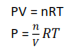
where p is the pressure in atmosphere, n is the number of moles, V, is the volume in cubic decimeter and T is the temperature in Kelvin. In this case R, the gas constant, has units are atmdm-3K-1mol-1
P = [gas]RT
Where [gas] is the concentration of the gas in moldm-3
Thus, at constant temperature, the pressure of a particular gas is proportional to concentration
i.e. P α [gas]
This means that for equilibrium
H2(g) + I2(g) ↔ 2HI(g)
we can either write
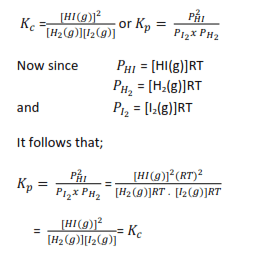
In this particular example Kp = Kc and neither nor has units, but this is not always the case;
Consider another reaction
N2(g) + 3H2(g) ↔ 2NH3(g)
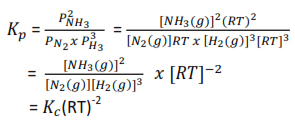
It implies that the numerical value of Kp is the same as that of Kc only where there are the same number of moles on each side of the Stoichiometric equation.
Although the SI unit of pressure is Nm-2, it is standard practice to use atmospheres as pressure unit in expressing Kp values.
In general, Kp = Kc (RT)∆n
where ∆n = number moles on the right of the equation minus number of moles on the left.
Example 4
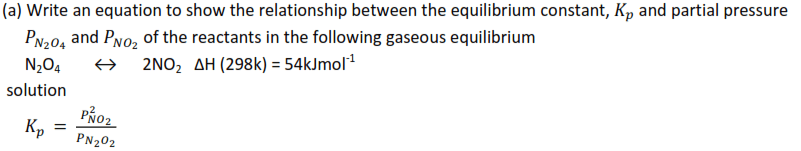
(b) State the effect, if any, on above equilibrium of
(i) increasing the pressure
Equilibrium shift from right to left leading to reduction in number of moles to fit in small volume.
(ii) raising the temperature . Give reason for your answer.
Equilibrium shifts from left to right to reduce on the increase in temperature since the forward reaction is endothermic.
(c) It was found that one dm3 of the gaseous mixture weighed 2.777g at 500C and pressure of 1.01 x 10-5Nm-2 (1 atmosphere)
Calculate
(i) The fraction of N2O4 that dissociated
solution
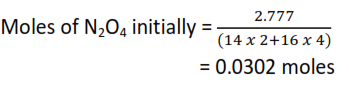
Moles at equilibrium
From ideal gas equation, PV = nRT
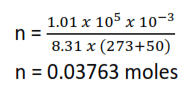
If α is the fraction ionizes
Then
[1 mole of a gas occupies 22.4dm3 at s.t.p]
N2O4 ↔ 2NO2
Initial moles, 0.0302 0
mole at equilibrium 0.0302(1-α) 0.0302(2α)
total moles at equilibrium
= 0.0302(1-α) + 0.0302(2α) = 0.03763mole
- α = 0.25
Mole
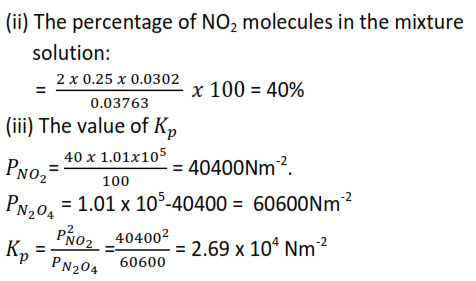
Factors affecting equilibrium
These are
1. Temperature
2. Pressure
3. Catalyst
4. Concentration
The effect of the above factors on equilibrium position for the equilibrium mixture can be explained qualitatively by Le Chatelier’s Principles that states that; ‘ if a system in equilibrium is subjected to a change, processes occur which tend to counteract the change imposed’.
Alternatively, ‘if the concentration of one of the reacting substance is altered, the equilibrium will shift in such a way as to oppose the change in concentration’
Effect of temperature
The effect of temperature on equilibrium depends on whether the reaction is endothermic or exothermic.
(i) For exothermic reactions (∆H0 negative) equilibrium position shifts to the left as temperature rise leading to a reduction in equilibrium constants
(ii) for endothermic reactions (∆H0 positive) equilibrium position shift the right as temperature increases leading to increase in the value of equilibrium constants.
Alternatively, the direction of change is given by Vant Hoff’s Law of mobile equilibrium: “if a system is in equilibrium raising the temperature will favor that reaction which is accompanied by absorption of heat, and lowering temperature will favor that reaction which is accompanied by evolution of heat.
NB. It must be emphasized that it is only temperature that can alter the equilibrium positions and values of equilibrium constants among the factors states above whereas other factors alter only the equilibrium positions.
Effect of a catalyst
Catalysts have no effect on the equilibrium constants Kc or Kp because they increase the rate of both forward and backward reaction in an equilibrium equally. However, they increase the rate at which equilibrium is attained.
Effect of concentration on equilibrium mixture
Variations in concentration of the reactant at constant temperature has no effect on the value of equilibrium constants Kp and Kc. However, if the concentration of one of the reacting substance in a reversible equilibrium is altered, the equilibrium will shift in such away as to oppose the change in concentration.
Effect of pressure changes on equilibrium
- Pressure has appreciable effects when at least one of the reactant or product in a reversible reaction is a gas because unlike liquids and solids, gases are compressible.
- The effect of pressure on equilibrium where reactants and/or products are in gaseous form depends on whether there is change in the number of molecules of the gases as the reaction proceeds from left to right.
(i) In general, for gaseous reaction in which there is a change in the number of molecules, increase in pressure favors a reaction which produces fewer molecules or number of moles of the products.
(ii) Pressure has no effect on those gaseous reactions in which there is no change in number of molecules as the reaction shifts from left to the right. e.g.
H2(g) + I2 (g) ↔ 2HI(g)
3. Generally high pressure increases the rate at which equilibrium is attained in gaseous mixture because it increases the rate of collision of the reacting molecules.
4. Pressure has no effect on numerical values of Kc and Kp at constant temperature although the equilibrium concentration of reactants and product can vary over a wide range.
Example 5
Hydrogen reacts with nitrogen to produce ammonia according the following equation
3H2(g) + N2(g) ↔ 2NH3(g)
(a) Write an expression for the equilibrium constant Kc (1 mark),
(b) State, giving reasons, what would happen to the value of the equilibrium constant when
(i) Pressure is increased at constant temperature (3marks)
There will be no effect on the equilibrium constant because only temperature varies the value of the constant.
(ii) argon is added to the reaction mixture at constant pressure (2marks)
There will be n effect on the equilibrium constant as long as temperature is constant.
(ii) Argon is added to the reaction mixture at constant volume (3marks)
This will increase pressure but there will be no change on the value of equilibrium constant of mixture because this value is not altered by pressure.
Watch this
Then this
For revision questions download PDF
Sponsored by The Science Foundation College +256 753 80 27 09
Compiled by Dr. Bbosa Science



Good work done
I’m always inspired by your words. Home & Kitchen
Great insights! Thanks for sharing. Real Madrid News
Simplify your admission process with MBBS Direct Admission in Uttar Pradesh, ensuring a seamless experience.
Get admission insights with MBBS Cutoff Of Private Medical Colleges in Maharashtra.
Access exclusive features and win exciting prizes with the Raja Luck App.
Enjoy a user-friendly user interface and smooth navigation on the 82 Lottery App.
Learn how to Earn backlinks without spammy techniques.
Sign up with Raja-Luck to access special gaming benefits and unique promotions.
Access high-speed networking and boosted security with Server Rental in Chennai.
I checked out throughout off-season and found Escort Service In Nainital still running with high standards. Worth discussing for year-round travelers!
Every round of 82 lottery deals something brand-new and interesting.
I enjoy that Youtube To MP3 Converter is ad-free and entirely free to use.