
Chemicals of life (A-level biology)

Chemicals of life
Overview
All cells are made up of a variety of substances some of which organic while others are inorganic. Water forms the largest component and is also a medium for all reactions in the cell. The other substance includes acids, bases, salts, vitamins, carbohydrates, lipids, and proteins. There are enzymes and nucleic acids which perform a variety of functions.
General objective
By the end of the topic, the learner should be able to describe the composition, structure, properties, and importance of inorganic and organic substances to the life of the organism.
Acids, bases, and salts
Specific objectives
The learner should be able to
- Describe the properties of acids and bases
- Explain the role of acids, bases, and salts in maintaining a stable internal environment for physiological processes.
Definition
An acid is a molecule or ion capable of donating hydrogen ion (proton or hydrogen ion H+) in solution. They neutralize alkalis, dissolves some metals with the liberation of hydrogen, and turns litmus red; typically, a corrosive or sour-tasting liquid of this kind:
A base is a substance that can accept a hydrogen ion (H+) from another substance
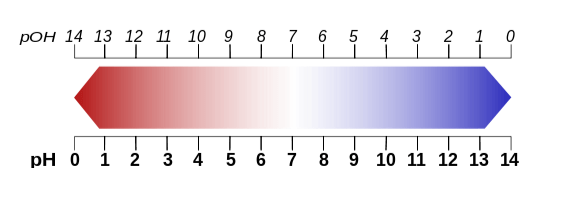
PH
In chemistry, pH is a numeric scale used to specify the acidity or basicity (alkalinity) of an aqueous solution. It is roughly the negative of the logarithm to base 10 of the concentration, measured in units of moles per liter, of hydrogen ions. More precisely it is the negative of the logarithm to base 10 of the activity of the hydrogen ion.
Here are some of the places that acids and bases can be found in the human body:
1. DNA is a complex NUCLEIC ACID found in cells that contains four unique nitrogen-based BASES: adenine, cytosine, guanine, and thymine. Differing combinations of these four bases determine the genetic characteristics of all human life.
2. AMINO ACIDS are the building blocks of proteins! The majority of amino acids consist of both a carboxylic acid (-COOH) and an amino (-NH2) functional group attached to the same tetrahedral carbon atom.
3. LACTIC ACID is an acid produced when sugars are processed by the body. The production of too much lactic acid can cause serious health issues and even death.
4. Vitamin C has the chemical name ASCORBIC ACID and Aspirin and other pain relief remedies are also organic acids. Here is the chemical structure of Aspirin:

5. BLOOD is naturally buffered to a pH of 7.35 – 7.45 in healthy humans. While our bodies naturally work to keep a healthy acid-base balance, problems can arise when acidic or basic compounds are too concentrated or not present in high enough concentrations.
The blood’s acid-base balance is precisely controlled because even a minor deviation from the normal range can severely affect many organs. The body uses different mechanisms to control the blood’s acid-base balance such as the release of CO2 from the lungs, adjusted kidney function for excretion of substances, and controlled buffering of blood via concentrations of the bicarbonate ion.
6. Acidosis and alkalosis are the two abnormalities of acid-base balance. In acidosis, the blood has too much acid (or too little base), resulting in a decrease in blood pH. In alkalosis, the blood has too much base (or too little acid), resulting in an increase in blood pH. Acidosis and alkalosis are not diseases, but rather are conditions that result for a variety of reasons.
Effects of unbalanced pH in the body
A person with mild metabolic acidosis may have no symptoms but usually experiences nausea, vomiting, and fatigue. Breathing becomes deeper and slightly faster (as the body tries to correct the acidosis by expelling more carbon dioxide). As the acidosis worsens, the person begins to feel extremely weak and drowsy and may feel confused and increasingly nauseated. Eventually, blood pressure can fall, leading to shock, coma, and death.
Systems responsible for maintenance of the acid-base balance
Several systems maintain constant pH. The list below is made according to order when they act:
1) Chemical buffering systems
Buffers react immediately – acute regulation.
They minimize pH changes that would otherwise occur in their absence. They do not correct pH deviations but only serve to reduce the extent of the change that would otherwise occur.
These buffers include the bicarbonate buffer system, the phosphate buffer system, and the protein buffer system. The capacity of buffers is not indefinite that is why chemical buffers act only in the short-term.
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO3– ), and carbon dioxide (CO2) in order to maintain pH in the blood and duodenum, among other tissues, to support proper metabolic function. Catalyzed by carbonic anhydrase, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3), which in turn rapidly dissociates to form a bicarbonate ion (HCO3– ) and a hydrogen ion (H+) as shown in the following reaction:
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3–
As with any buffer system, the pH is balanced by the presence of both a weak acid (for example, H2CO3) and its conjugate base (for example, HCO3– ) so that any excess acid or base introduced to the system is neutralized
2) Respiratory system
Respiration reacts in 1-3 minutes. The respiratory system regulates carbon dioxide. Respiration is able to change pCO2 by its elimination or retention. Respiratory center is in the brainstem.
3) Kidneys
Kidneys react in hours-days. In the kidney, carbon dioxide reacts with water to form hydrogen ions (H+) and hydrogen carbonate (HCO3–)
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3–
When the pH is low the collecting duct and distal convoluted tubules secrete hydrogen ions and retain hydrogen carbonate ions. Therefore, acidic, or that is to say urine with low pH, will be excreted in that case.
Conversely, if blood is too alkaline, then the collecting duct can secrete bicarbonate into urine and retain H+ lowering the pH of blood; therefore cause the urine to become more alkaline.
4) Liver
The liver is a pivotal organ of the energetic metabolism it also has an important influence on the acid-base balance. The liver is the most important tissue where ammonium is detoxified in both (a) urea cycle, and (b) glutamine synthesis. Which one of these fates of ammonium is favored closely depends on the status of the acid-base balance:
(a) NH4+ → urea + 2 H+ → acidification of the body
CO2 + 2 NH4+ → CO(NH2)2 + 2 H+ + H2O
H+ + HCO3– → H2O + CO2 (consumption of bicarbonate–)
(b) NH4+ → glutamine synthesis → H+ is not produced, glutamine is taken up by the kidneys. In the kidney is H+ excreted as NH4+
Water
Specific objectives
The learner should be able to
- Describe the molecular structure of water
- State the function of water
- Explain the importance of water as a solvent
- Relate the water properties to its role in the life of an organism
- To test for water
- Determine water content in tissue by using dry weight method.
Water
This is the most abundant compound, typically making up of 60-95% the fresh mass of an organism.
Structure of water
Water is made of hydrogen and oxygen.
2H2(g) + O2 (g) → 2H2O(l)
The H2O molecule is electrically neutral, but polar because the positive and negative charges are not distributed uniformly. This lead to partial positive charges on hydrogen atoms and a partial negative charge on the oxygen atom
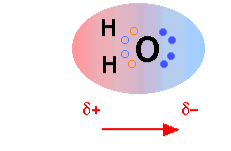
In liquid and solids, these partial charges attract to form hydrogen bonds
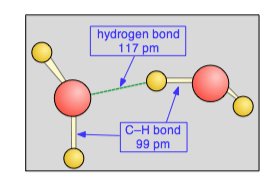
The hydrogens are responsible for the high melting and boiling point and strong surface tension of water.
Uses of water
- It makes up structures of the organism
- It is a solvent
- It is a reagent in the hydrolysis reaction
- Provide support for aquatic organism
- It is a medium of fertilization through which gametes swim.
- Medium for the removal of waste products
- Temperature control
- Hearing and balance as endolymph
Uses of water to the plants
- Aid seed dispersal
- Provide support to herbaceous plants
- Breaks up the testa of seed during germination
- Reagent in photosynthesis
- Loss of water through the leaves cools the plant.
- Medium of fertilization
Uses of water to the animals
- A medium of transport
- Evaporation cools the animal
- Lubricates joints, eyes, lungs
- A constituent of protecting fluids such as tears, mucus.
Adaptation of Water to its function
- Water is the universal solvent:
Polar and ionic substances have electrostatic charges, so they are attracted to the charges on water molecules and dissolve readily. Non-polar substances, such as oil, do not dissolve in water, as they do not have charged molecules. When a salt dissolves in water, the ions separate and a layer of water molecules form around the ions. These layers prevent the ions or polar molecules from clumping together, keeping the particles in solution.
- Water has a high surface tension:
At an interface between air and water, a water molecule on the surface forms hydrogen bonds with other molecules around and below it, but not with air molecules above it. The unequal distribution of bonds produces a force called surface tension; this causes the water surface to contract and form a surprisingly tough film or ‘skin’ enabling small animals like insects to walk over. It also protects blood capillaries of gill filaments from bursting.
- Ice floats on water:
Water is at its most dense at 4oC. When water freezes the hydrogen bonds between the molecules form a rigid lattice, that holds the molecules further apart than in liquid water. Ice, having expanded when freezing, is less dense than its liquid counterpart and so floats on water. This protests water from further freezing because ice insulates the surface of the water.
- Water is adhesive and cohesive:
Water is ‘wet’ because it sticks to things. This is because its molecules can form hydrogen bonds with other polar substances. This is called adhesion. The attraction between molecules of similar substances is called cohesion. In this way, water molecules stick together which allows water to enter and move along very narrow spaces, in a process called capillarity. This enables water to ascend in the xylem of a tall tree.
- Important thermal properties:
Water has a high specific heat capacity meaning that it needs to gain a lot of energy to raise its temperature. Conversely, it also needs to lose a lot of energy to lower its temperature. Water’s specific heat capacity is 4.2 kJ/g/oC. Thus, this minimizes the increase or decrease in temperature on hot days.
Water has a high latent heat of vaporization which means a lot of energy is required to evaporate it. When it evaporates, water draws thermal energy out of the surface it’s on, which can be observed in sweating. Thus, sweating cools the body.
Water also has a high latent heat of fusion meaning that at 0oC water must lose a lot of thermal energy before it freezes, thus liquid water can reach temperatures of down to -10oC before it forms ice. This implies too much heat loss is required to freeze the water body.
- Other physical properties of water:
It is transparent to sunlight. This allows animals to see in water also photosynthesis in aquatic plants.
It has a relatively high density compared to air, this support aquatic animals while swimming.
It is difficult to compress. Thus, support aquatic animals while swimming.
It conducts electricity (when it contains dissolved ions) to enable the conduction of heat, keeping water hot.
Inorganic compounds/mineral salts
- Inorganic salts include those needed in large amounts such as salts of Na+, Mg2+, Cl–, K+ and those needed in trace amounts such as manganese, iron, cobalt, copper, zinc, boron, aluminium, silicon, vanadium, molybdenum and iodine
Functions of inorganic salts
- They are components of proteins, e.g. nitrogen, phosphorus, and Sulphur.
- They are components of tissues; e.g. calcium and phosphorus are components of bones
- They are constituents of enzymes; e.g. copper and iron.
- They are metabolic activators, for example, magnesium activates enzymes in phosphate metabolism.
- They are constituent of pigments, for example, iron in hemoglobin and magnesium in chlorophyll.
- They are determinants of anion-cationic balance in the cell example Na+, Cl–, and K+.
- They are determinants of osmotic pressure, e.g. Na+, Cl–, and K+.
Carbohydrates
Specific objectives
The learner should be able to:
- Describe the structure and components of various carbohydrates
- Explain the properties of carbohydrates.
- Explain the functions of carbohydrates in organisms.
- Describe the condensation of carbohydrates
- Describe the hydrolysis of carbohydrates
- Identify the various categories of carbohydrates.
Carbohydrates are food substances with a general formula (CH2O)n where n is a natural number.
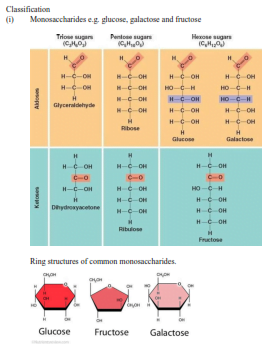
Sources: honey, fruits
Properties
- They are sweet
- Are soluble in water
- They reduce blue copper II ions to red precipitates in an alkaline medium.
Testing for reducing sugars
When boiled with Benedict’s or Fehling’s solutions the color changes from blue to green to yellow to oranges ppt.
(ii) Disaccharides
They are made of two simple sugars by condensation
Illustration
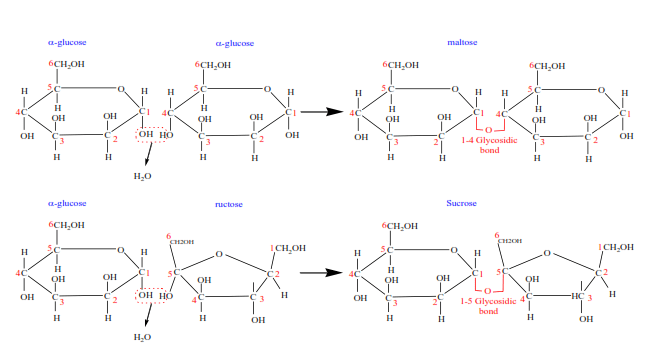
Examples of disaccharides and their composition
| Disaccharides | Composition | Source |
| Maltose | glucose + glucose | malt |
| Lactose | Glucose + galactose | milk |
| Sucrose | Glucose + fructose | Sugar cane Sugar beets |
| Cellobiose | Glucose + glucose | wood |
- Maltose, lactose, and sucrose are sweet and are referred to as sugars. Maltose and lactose are reducing sugars whereas sucrose is not and referred to as non-reducing sugar.
Testing for non-reducing sugars
- When boiled with Benedict’s solution or Fehling’s’ solution, the color remains blue.
- When boiled with HCl, the solution cooled, neutralized by NaOH, boiled Benedict’s or Fehling’s solution, the color changes from green to yellow to orange.
HCl hydrolyzes non-reducing sugars
NaOH neutralizes the excess acid because Benedict’s or Fehling’s solution does not work in acidic medium.
Polysaccharide (CH2O)n
These are made of very many monosaccharides per unit molecule e.g. starch and cellulose
Testing for starch
It changes the color of iodine black or blue.
Functions of carbohydrates
- Glucose, galactose, and fructose are oxidized to release energy in the body
- Glyceraldehyde is an intermediate molecule in photosynthesis.
- Ribose is a component nucleotide.
- Sucrose is a form in which carbohydrates are transported in plants
- Lactose is a source of energy in milk
- Storage of energy (starch in plants, glycogen in animals, inulin is some plants like Dahlia)
- Formation of cellular structures (cellulose in plant cell walls, chitin is
Lipids
Specific objectives
The learner should be able to:
- Describe the structure and components of lipid molecules.
- State the properties of lipids.
- Explain the functions of lipids in organisms.
- Describe the structure of steroids
- Explain the effects of lipids and steroids to organisms
- Describe the condensation of fatty acids and glycerol to form lipids
- Describe the hydrolysis of lipids to fatty acids and glycerol
- Compare waxes and lipids
- State importance of cholesterol in an organism
- To carry out tests to identify lipids on food and extracts.
Lipids include natural fats and oil. Fats are solids at room temperature while oils are liquids
Structure of lipids
Lipids are ester of glycerol and fatty acids

Table of nature and occurrence of some fatty acid
| Name of fatty acid | formula | Saturated/unsaturated | occurrence |
| Butyric acid | C3H7COOH | Saturated | Butterfat |
| Linoleic | C17CH31COOH | unsaturated | Linseed oil |
| Oleic | C17H33COOH | unsaturated | All fats |
| Palmitic | C15H31COOH | Saturated | Animal and vegetable fats |
| Stearic | C17H35COOH | Saturated | Animal and vegetable fats |
| arachidic | C19H39COOH | Saturated | Peanut |
| Ceritic | C25H51COOH | Saturated | Wool oil |
Uses of lipids
Structural functions of lipids
- Make up the cell membrane
- Protection: lipids are constituents of the waxy cuticle of plants and insects
- Lipids are water repellant thus prevent water loss from or entry into an animal skin
- Their spongy nature protects delicate organs as shock absorbers.
- Being bad conductors, they reduce water loss from the body when deposited beneath the skin for insulation
- Storage; they are better storage compounds than carbohydrates due to high calories value, due to high hydrogen content, they are light, insoluble in water, compact to fit in a small volume, and are easily used when required.
Physiological functions of lipids
- Source of metabolic water
- Store fat-soluble vitamins (ADEK)
- Source of metabolic water
- Raw materials for hormones
Testing for lipids
- They form a translucent mark on a paper that does not disappear when the paper is dried on a flame.
- Emulsion test
When 2cm3 of fats or oil are dissolved in 2cm3 of absolute ethanol followed by water, a white cloudy suspension is formed.
- Sudan III
When a few drops of Sudan III are added to a mixture of 2cm3 of water and 2 cm3 of oil and shaken, a red-stained oil layer separates out.
Phospholipids.
These are lipids in which fatty acid is replaced by phosphoric acid. The phosphoric acid attracts water (hydrophilic) whereas the rest of the group repel water (hydrophobic). This property enables them to form a cell membrane.
Waxes
Waxes are formed by combination with alcohol other than glycerol. This alcohol is much larger than glycerol, and therefore waxes have a more complex chemical structure. The main role of waxes is waterproofing plants and animals although, they form storage compounds in a few organisms, e.g., castor oil and in fish.
Advantage of storing fats over carbohydrate
(i) Has high energy content than carbohydrates
(ii) It is lighter
(iii) It is compact and requires less space
(iv) It is a raw material for hormones
(v) Insoluble in water that they have low osmotic value
Proteins
Specific objectives
The learner should be able to:
- Describe the structure and composition of proteins.
- Describe the properties of proteins
- Explain the importance of proteins
- Explain the functions of proteins in organisms
- Describe the condensation of amino acids to form proteins.
- Describe the hydrolysis of proteins to amino acids
- The effect of heat/temperature changes on proteins
- To carry out the test and identify proteins on food /extracts.
Proteins
These are classified into two groups
- Structural proteins: insoluble proteins that makeup body structures like bones and muscles. Fibrinogen is a soluble structure protein used in blood clotting.
- Globular proteins are soluble proteins such as enzymes, antibodies, hormones, and so on.
Composition of proteins
The basic unit of proteins are amino acids
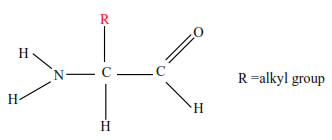
There are about 22 different amino acids in the body of which isoleucine, leucine, methionine, phenylamine, proline, threonine, and valine cannot be synthesized in the human body and they are referred to as essential amino acids.
Amino acids unite to form proteins through the formation of peptide bonds by a condensation reaction in which a water molecule is eliminated.

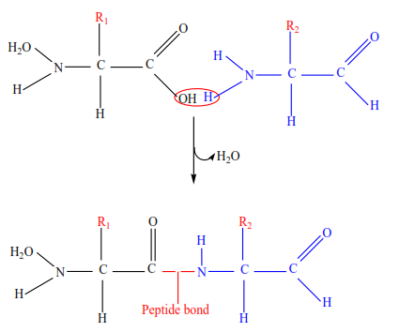
Uses of proteins
(i) Make up structures, e.g., collagen makes up connective tissues.
(ii) Makeup enzymes such as catalyze and amylase.
(iii) Are constituents of hormones such as insulin
(iv) Are constituents of antibodies that protect the body from foreign particles.
(v) Makeup muscles such as myosin and actin
(vi) They are storage food e.g. egg white
(vii) Constitute toxins such as snake venom for protection.
Testing for proteins
- They coagulate on heating
- They coagulate on the addition of Melon’s reagent and on heating they form a pink precipitate.
- They form a purple color when mixed with an equal amount of NaOH followed by 3 drops of copper sulfate solution.
Vitamins
Specific objects
The learner should be able to:
- State the type of vitamins i.e., water-soluble and fat-soluble; essential and nonessential.
- State the importance of vitamins in organism
- Test for vitamin C.
- Demonstrate the effect of over boiling vegetables
- Demonstrate the effects of storage on the quality of fresh food.
Vitamins are complex organic compounds present in very small quantities in natural food which are essential for a good healthy body and maintains its normal metabolic activities. Some vitamins are fat-soluble (ADEK) while others are not.
Vitamin C / ascorbic acid
Sources: citrus fruits, green vegetables, potatoes, tomatoes, etc.
The function of vitamin C
Concerned with the metabolism of connective tissues and the production of strong skin.
Deficient disease: anemia and scurvy: the germs bleed, wounds fail to heal.
Testing for Vitamin C
It decolorizes DCPIP.
Other vitamins and their deficient diseases
| Vitamin | |
| A | Night blindness |
| K | Delayed clotting |
| E | Reduced fertility in rats |
Functions of vitamins protect the body against diseases
(i) formation of coenzymes that facilitate enzyme reactions
(ii) blood clotting
(iii) components of visual pigment
For questions and answers download PDF. For corrections and addition send comments.

The notes are so helpful to me thanks very much
Helpful
It is so helpful. Please I also need the questions and answers of chemicals of life. Thank you
It’s so helpful. Please I also need question and answers of chemicals of life.thank you
Good content
The notes are very good and they ate easily understandable even to learners or fellow students who tend not focus alot on notes
So helpful
Marvelous
Nice work
I really like and appreciate your article post.Thanks Again.
Your writing has a real personal touch. Water Bottle
Your blog is a source of inspiration for me. Sports News
Plan effectively with insights from the MBBS Cutoff Of Private Medical Colleges in Orissa.
Achieve hassle-free enrollment with MBBS Admission Through Management/Nri Quota in Tamil Nadu.
thanks for the wonderful biology……i request that u send mi alevel syallabus on mi email……..