
Chemicals of life (O-level biology)

Chemicals of life
Overview
All cells are made up of a variety of substances some of which organic while others are inorganic. Water forms the largest component and is also a medium for all reactions in the cell. The other substance includes acids, bases, salts, vitamins, carbohydrates, lipids, and proteins. There are enzymes and nucleic acids which perform a variety of functions.
Water
This is the most abundant compound, typically making up of 60-95% the fresh mass of an organism.
Uses of water
- It makes up structures of an organism
- It is a solvent
- It is a reagent in the hydrolysis
- Provide support for aquatic organism
- It is a medium of fertilization through which gametes swim.
- Medium for the removal of waste products
- Temperature control
- Hearing and balance as endolymph
Uses of water to the plants
- Aid seed dispersal
- Provide support to herbaceous plants
- Breaks up the testa of seed during germination
- Reagent in photosynthesis
- Loss of water through the leaves cools the plant.
- Medium of fertilization
Uses of water to the animals
- A medium of transport
- Evaporation cools the animal
- Lubricates joints, eyes, lungs
- A constituent of protecting fluids such as tears, mucus.
Carbohydrates
Carbohydrates are food substances with a general formula (CH2O)n where n is a natural number. They are energy giving foods.
Classification
- Monosaccharides e.g. glucose, galactose, and fructose
Sources: honey, fruits
Properties
- They are sweet
- Are soluble in water
- They reduce blue copper II ions to red precipitates in an alkaline medium.
Testing for reducing sugars
When boiled with Benedict’s or Fehling’s solutions the color changes from blue to green to yellow to oranges ppt.
- Disaccharides
They are made of two simple sugars as shown in the table below
.
| Disaccharides | Composition | Source |
| Maltose | glucose + glucose | malt |
| Lactose | Glucose + galactose | milk |
| Sucrose | Glucose + fructose | Sugar cane Sugar beets |
| Cellobiose | Glucose + glucose | wood |
Testing for non-reducing sugars
- When boiled with Benedict’s solution or Fehling’s’ solution, the color remains blue.
- When boiled with HCl, the solution cooled, neutralized by NaOH, boiled Benedict’s or Fehling’s solution, the color changes from green to yellow to orange.
HCl hydrolyzes non-reducing sugars
NaOH neutralizes the excess acid because Benedict’s or Fehling’s solution does not work in acidic medium.
Polysaccharide (CH2O)n
These are made of very many monosaccharides per unit molecule e.g. starch and cellulose
Testing for starch
It changes the color of iodine black or blue.
Functions of carbohydrates
- Glucose, galactose, and fructose are oxidized to release energy in the body
- Glyceraldehyde is an intermediate molecule in photosynthesis.
- Ribose is a component nucleotide.
- Sucrose is a form in which carbohydrates are transported in plants
- Lactose is a source of energy in milk
- Storage of energy (starch in plants, glycogen in animals, inulin is some plants like Dahlia)
- Formation of cellular structures (cellulose in plant cell walls, chitin is
Lipids
Lipids are made of fats and oils. Fats are solids at room temperature while oils are liquids at room temperature
Uses of lipids
Structural functions
- Makeup cell membrane
- Protection: lipids are constituents of the waxy cuticle of plants and insects
- Lipids are water repellant thus prevent water loss from or entry into an animal skin
- Their spongy nature protects delicate organs as shock absorbers.
- Being bad conductors, they reduce water loss from the body when deposited beneath the skin for insulation
- Storage; they are better storage compounds than carbohydrates due to high calories value, due to high hydrogen content, they are light, insoluble in water, compact to fit in a small volume, and are easily used when required.
Physiological functions
- Source of metabolic water
- Store fat-soluble vitamins (ADEK)
- Source of metabolic water
- Raw materials for hormones
Testing for lipids
- They form a translucent mark on the paper that does not disappear when the paper is dried on a flame.
- Emulsion test
When 2cm3 of fats or oil are dissolved in 2cm3 of absolute ethanol followed by water, a white cloudy suspension is formed.
- Sudan III
When a few drops of Sudan III are added to a mixture of 2cm3 of water and 2 cm3 of oil and shaken, a red-stained oil layer separates out.
Advantage of storing fats over carbohydrate
- Has high energy content than carbohydrates
- It is lighter
- It is compact and requires less space
- It is a raw material for hormones
- Insoluble in water that they have low osmotic value
Proteins
These are classified into two groups
- Structural proteins: insoluble proteins that makeup body structures like bones and muscles. Fibrinogen is a soluble structure protein used in blood clotting.
- Globular proteins are soluble proteins such as enzymes, antibodies, hormones, and so on.
Composition of proteins
The basic unit of proteins is amino acids

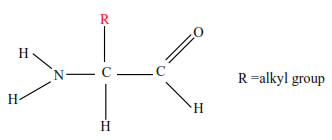
There are about 22 different amino acids in the body of which isoleucine, leucine, methionine, phenylamine, proline, threonine, and valine cannot be synthesized in the human body and they are referred to as essential amino acids.
Uses of proteins
- Makeup structures, e.g., collagen make up connective tissues.
- Makeup enzymes such as catalyze and amylase.
- Are constituent of hormone such as insulin
- Are constituents of antibodies that protect the body from foreign particles.
- Makeup muscles such as myosin and actin
- They are storage food e.g. egg white
- Constitute toxins such as snake venom for protection.
Testing for proteins
- They coagulate on heating
- They coagulate on the addition of Melon’s reagent and on heating they form a pink precipitate.
- They form a purple color when mixed with an equal amount of NaOH followed by 3 drops of copper sulphate solution.
Vitamins
Specific objects
The learner should be able to:
- State the type of vitamins i.e., water-soluble and fat-soluble; essential and nonessential.
- State the importance of vitamins in organism
- Test for vitamin C.
- Demonstrate the effect of over boiling vegetables
- Demonstrate the effects of storage on the quality of fresh food.
Vitamins are complex organic compounds present in very small quantities in natural food which are essential for good healthy body and maintains its normal metabolic activities. Some vitamins are fat-soluble (ADEK) while others are not.
Vitamin C / ascorbic acid
Sources: citrus fruits, green vegetables, potatoes, tomatoes, etc.
The function of vitamin C
Concerned with the metabolism of connective tissues and the production of strong skin.
Deficient disease: anemia and scurvy: the germs bleed, wounds fail to heal.
Testing for Vitamin C
It decolorizes DCPIP.
Other vitamins and their deficient diseases
| Vitamin | |
| A | Night blindness |
| K | Delayed clotting |
| E | Reduced fertility in rats |
Functions of vitamins
- protect the body against diseases
- formation of a coenzyme that facilitates enzyme reactions
- blood clotting
- components of visual pigment
For questions and answers download PDF
Sponsored by The Science Foundation College +256 753 802709
Compiled by Dr. Bbosa Science

Thanks for being a source of wisdom. Baby Products
I’m always learning something new. Indian Cricket
Explore comprehensive details about the MBBS Fees Structure in Delhi, including tuition and additional costs.
Learn how MBBS Admission Through Management/Nri Quota in Gujarat helps aspiring medical students.
The Raja Luck App Download gives you access to premium games.
I’m delighted to apply what I gained from Link-Building For Beginners. Thanks!
Scale up cloud facilities with high-end Server Rental in Bengaluru.
Such lovely descriptions of Nainital! I improved my journey with friendship from Escort Service In Nainital and had a terrific time.
The interface of bdg win is smooth, making it easy to play anytime.
I love that Youtube To MP3 Converter is ad-free and completely complimentary to use.
Discover the gamers and key methods in the **Sunrisers Hyderabad Team 2025**, featuring leading performers and emerging skills.