
Chemistry of period 3 elements (A-level inorganic chemistry)

Chemistry of period 3 elements
This chapter compares the Chemistry of the elements in period 3 of the periodic table. Each element in a period provides representative Chemistry of the group into which it exists.
The elements of period 3 are Na, Mg, Al, Si, P, S, Cl and Ar.
Electronic structure
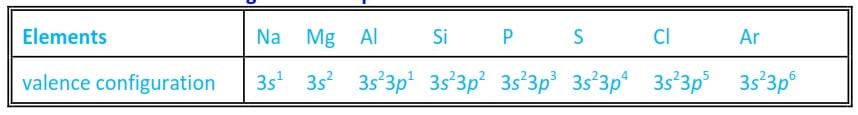
For each of these elements, the total number of electrons in the outermost shells is equal to the group number in which it belongs (table 6.1).
Table 1 The electron configurations of period 3 elements
Physical properties
Sodium, magnesium and aluminium are metals whose melting points increase in that order due to increase in the number of electrons contributed to form metallic bonds. Silicon, phosphorus, sulphur, chlorine and argon are nonmetals. Silicon, phosphorus and sulphur are solids while chlorine and argon are gases at room temperature.
Reactions of the elements
a) Reaction with water
Reactivity with water decreases across the period because there is a decrease in electropositivity of these elements across the period.
(i) Sodium reacts vigorously with cold water to form sodium hydroxide and hydrogen gas.
2Na (s) + 2H2O (l) → 2NaOH (aq) + H2 (g)
ii) Magnesium slowly with cold water to form magnesium hydroxide but reacts with steam to form magnesium oxide and hydrogen gas.
Mg (s) + 2H2O (l) → Mg(OH)2 (aq) + H2 (g)
Mg (s) + H2O (g) → MgO (aq) + H2 (g)
iii) Aluminium reacts with steam very slowly to form aluminium hydroxide and hydrogen gas.
2Al (s) + 6H2O (l) → 2Al(OH)3 (s) + 3H2 (g)
iv) Silicon, phosphorus, sulphur and argon have no reaction with water.
v) Chlorine reacts with water to form chloric (I) acid and hydrochloric acid.
Cl2 (g) + H2O (l) → HOCl (aq) + HCl (aq)
b. Reaction with NaOH
(i) Sodium and magnesium have no reaction with sodium hydroxide because they are metals and magnesium falls below sodium in the reactivity series.
(ii) Aluminium reacts with aqueous NaOH to produce hydrogen and a complex salt -sodium aluminate.
2Al (s) + 2NaOH (aq) + 6H2O (l) → 2NaAl(OH)4 (aq) + 3H2 (g)
This is because aluminium is amphoteric, i.e., possesses both acidic and basic properties.
ii) Chlorine reacts with cold dilute sodium hydroxide to form sodium chlorate (I), sodium chloride and water.
Cl2 (g) + 2NaOH (aq) → NaOCl (aq) + NaCl (aq) + H2O (l)
Sodium hypochlorate (I) is used as a bleaching and antiseptic agent .
Chlorine reacts with concentrated sodium hydroxide to form sodium chlorate (V), sodium chloride and water.
3Cl2 (g) + 6NaOH (aq) → NaClO3 (aq) + 5NaCl (aq) + 3H2O (l)
iii) Phosphorus reacts with hot concentrated NaOH to produce phosphine
P4 (s) + 3OH– (aq) + 3H2O (l) → PH3 (g) + 3H2PO2– (aq)
iv) Na, Mg, Si, S have no reaction with sodium hydroxide.
c. Reaction with HCl.
Reactivity decreases across the period due to decrease in metallic properties.
i) Na, Mg, Al react to form H2 and metal salts.
2Na (s) + 2HCl (aq) → 2NaCl (aq) + H2 (g)
Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
2Al (s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2 (g)
ii) Si, P, S, Cl have no reaction with hydrochloric acid.
d) Oxides
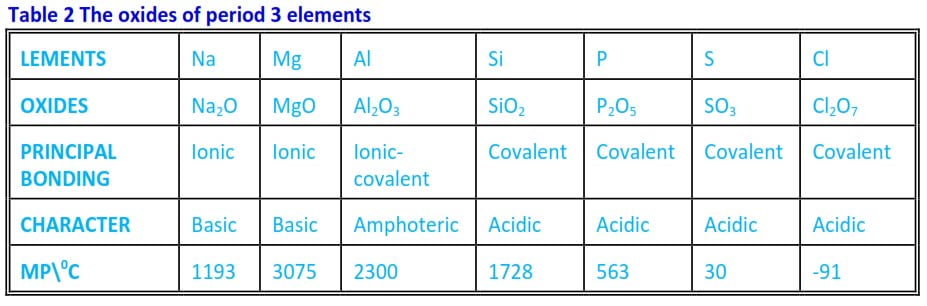
They are formed by reacting the elements with oxygen and the formulae of oxides are given in table 6.2.
Table 2 The oxides of period 3 elements
Trial 1
(a) Complete the table below ( marks)
| Element | Formulae of oxides | Type of bonding in oxide | Structures of oxides |
| Al | |||
| Si | |||
| P |
(b) Write an equation for the reaction between the oxide of aluminium and sodium hydroxide (1½)
a. Physical properties of oxides of period 3 elements
i) Across the period there is a gradual change in the character of oxides from strongly basic to strongly acidic. Al2O3 is an amphoteric oxide, i.e., possesses both basic and acidic properties.
ii) – Na2O, MgO and Al2O3 have high melting points due to the strength of ionic bonding.
- The melting point of magnesium is higher than that of Na2O because MgO, stronger electrostatic forces since Mg2+ has 2 positive charges, secondary, it has higher molecular mass.
- The melting point of Al2O3 is lower than that of MgO because Al2O3 has bigger covalent character due to high polarizing power of Al3+ ions.
- SiO2, although covalent, has a high melting point because the crystal of SiO2 is a giant structure in which silicon and oxygen atoms are bonded together by strong single covalent bonds.
- P2O5 (white solid) consists of P4O10 molecules; these contain electric dipoles due to electron displacements in their bonds and are quite strongly attracted to each other. This is why its melting point is high compared with that of P4O10 which consists of simple SO3 molecules.
- Cl2O7 has a low melting point because the molecules are held together by the weak van der Waals forces.
b. Reaction
I) The reaction of oxides of period 3 elements with water.
- Na2O and MgO react with water to form hydroxides.
Na2O (s) + H2O (l) → 2NaOH (aq)
MgO(s) + H2O (l) → Mg(OH)2 (aq)
- Al2O3 and SiO2 have no reaction with water because they are insoluble oxides.
- P2O5, SO3, Cl2O7 are acid anhydrides, i.e., react with water to form phosphoric, sulpuric and perchloric acids respectively.
P2O5 (s) + 3H2O (l) → 2H3PO4 (aq)
SO3 (s) + H2O (l) → H2SO4 (aq)
Cl2O7 (g) + H2O (l) → 2HClO4(aq)
II) The reactions of period 3 oxides with NaOH.
- Na2O, MgO are basic, therefore, have no reaction with water.
- Al2O3 being amphoteric reacts with dilute NaOH to produce sodium aluminate and water.
Al2O3 (g) + 2NaOH (aq) → 2NaAlO2(aq) + H2O (l)
- SiO2 reacts to form sodium silicate and water.
SiO2 (s) + 2NaOH (aq) → Na2SiO3 (aq) + H2O (l)
- P2O5, SO3 and Cl2O7 react to form salts and water
SO3 (g) + 2NaOH (aq) → Na2SO4 (aq) + H2O (l)
P2O5 (s) + 2NaOH (aq) → 2NaPO3 (aq) + H2O (l)
Cl2O7 (g) + 2NaOH (aq) → 2NaClO4 (aq) + H2O (l)
Trial 2
Write ionic equations for the reactions between sodium hydroxide and (1½ mark each)
- Silicon (IV) oxide.
- Lead (II) oxide.
- Aluminium oxide.
e. Chlorides of period 3 elements
These are formed by reacting the elements with chlorine gas and the physical properties of their chlorides are shown in table 3.
Table 3 The chlorides of period 3 elements
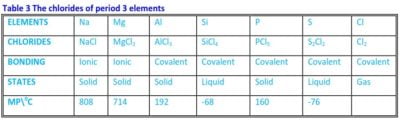

NaCl and MgCl2 have high melting points due to the strength of the ionic bonding. AlCl3 has a fairly high melting point because, in the solid-state, it consists of Al2Cl6 molecules and not simple AlCl3. These molecules are produced through dative bonding between Al and Cl in the Al2Cl6 molecules.

SiCl4 and S2Cl2 are liquids and consist of respective simple SiCl4 and S2Cl2 molecules and in the solid-state the molecules are held by weak van der Waals force which explains their very low melting points. PCl5 a pale yellow solid has a fairly high melting point because the solid undergoes partial ionisation.
2PCl5 ↔ PCl4+ + PCl6–
Trial 3
Sodium chloride melts at 8000C whereas aluminium chloride sublimes at 1800C. Explain the following observations: (3marks)
Boiling points of chlorides of period 3 elements
The table below shows the boiling points of the chlorides of period 3 elements.
|
Element |
Na |
Mg |
Al |
Si |
P |
S |
Cl |
|
Atomic number |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
|
Formula of the chloride |
NaCl |
MgCl2 |
AlCl3 |
SiCl4 |
PCl3 |
S2Cl2 |
Cl2 |
|
Boiling point (oC) |
1465 |
1418 |
180 |
57 |
76 |
136 |
-35 |
A graph of boiling points of the chlorides against atomic number of the elements. 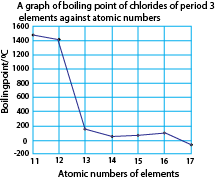
Explanation
NaCl and MgCl2 have high boiling points due to strong the ionic bonding.
Aluminium chloride, silicon chloride, phosphorus chloride, disulphur dichloride and chlorine have low boiling points because their molecules are held by weak molecular forces.
AlCl3 has a fairly high boiling point because in the liquid state, it consists of Al2Cl6 molecules and not simple AlCl3. These molecules are produced through dative bonding between Al and Cl in the Al2Cl6 molecules.

Disulphur dichloride has relatively high boiling point due to its high molecular mass that increases the strength of the molecular forces
The reaction of chlorides of period 3 with water.
NaCl and MgCl2 have no reaction with water but simply dissociate into ions.
NaCl (s) + (aq) → Na+ (aq) + Cl– (aq)
MgCl2 (s) + (aq) → Mg2+ (aq) + 2Cl– (aq)
ii) AlCl3, SiCl4, PCl5, Cl2 are hydrolyzed by water. However, the extent of the hydrolysis of these chlorides varies across the period.
AlCl3 is slightly hydrolyzed liberating HCl and Al(OH)3.
AlCl3 (s) + 3H2O (l) → Al(OH)3(s) + 3HCl (aq)
However, when aluminium chloride is added to water, it reacts exothermically to give hydrated aluminium ions, [ Al (H2O)6]3+ and chloride ions, Cl–. The energy needed to break the Al-Cl covalent bond is derived from the high enthalpy of hydration of small highly charged Al3+ ions.
Al2Cl6 (s) + 12 H2O (l) → 2[Al(H2O)6]3+(Cl–)3 (aq)
covalent ionic
Hydrated aluminium chloride is readily soluble in water yielding
[Al(H2O)6]3+ (aq) + 3Cl– (aq) ions
SiCl4, PCl5, and S2Cl2 are completely hydrolyzed liberating hydrogen chloride.
PCl5 (s) + 4H2O (l) → 5HCl (aq) + H3PO4 (aq) [phosphoric acid]
SiCl4 (l) + 3H2O (l) → 4HCl (aq) + H2SiO3 (aq) [silicic (IV) acid]
2S2Cl2 + 3H2O (l) → 4HCl (aq) + H2SO3 (aq) + 3S (s)
Hydrogen chloride under moist conditions appears as white fumes. For this reason, SiCl4, PCl5, S2Cl2 fume in moist air since their hydrolysis leads to formation of HCl.
Trial 4
The melting points of the chlorides of some elements are given in table 6.4 below.
Table 4 Melting points of chlorides
| chlorides | MgCl2 | FeCl3 | PCl5 |
| Mp/0C | 712 | 282 | -112 |
| Type bonding |
(a) State the type of bonding that exists in each of the chlorides in the table above. (1 ½ marks)
(b) State what would be observed and write an equation for the reaction that takes place when water is added to each of the chlorides in the table above. (2½ marks each)
f, The hydrides of period 3 elements.
The hydrides formed by period 3 elements are NaH, MgH2, AlH3, SiH4, PH3, H2S, HCl. These hydrides are formed by action of hydrogen on the elements.

NaH and MgH2 have high melting and boiling points due to the strength of ionic bonds. H2S and HCl have relatively higher melting and boiling points than SiH4 and PH3 because their molecules are polar molecules and are held together by hydrogen bonds whereas the molecules of SiH4 and PH3 are non-polar and therefore, held together by the weak van der Waals forces.
Reactions
The reaction of hydrides of period 3 elements with water.
Due to the high polarity of the bonds in the hydrides NaH and MgH2, they react with water readily producing hydrogen and the metal hydroxides.
NaH (s) + H2O (l) → NaOH (aq) + H2 (g)
MgH2 (s) + 2H2O (l) → Mg(OH)2 (aq) + 2H2 (g)
AlH3, SiH4, and PH3 have no reaction with water due to lack of polarity in their bonds.
H2S and HCl dissociate in water producing acids.
H2S (g) + 2H2O (l) ↔ H3O+ (aq) + HS– (aq) + H2O (l) ↔ 2H3O+ (aq) + S2- (aq)
HCl (g) + H2O (l) → H3O+ (aq) + Cl– (aq)
Reactions of hydrides of period 3 elements with hydroxides (NaOH)
HCl and H2 S react with hydroxides to form salts.
HCl (g) + OH– (aq) → Cl– (aq) + H2O (l)
H2S (g) + OH– (aq) → HS– (aq) + H2O (l)
then HS– (aq) + OH– (aq) → S2- (aq) + H2O (l)
Reaction of period 3 element with HCl.
NaH and MgH2 react to form hydrogen and metal salts.
NaH (s) + HCl (aq) → NaCl (aq) + H2 (g)
MgH2 (s) + 2HCl (aq) → MgCl2 + 2H2 (g)
Trial 5
The elements contained in the third short period of the periodic table, given in alphabetic order are; aluminium, argon, chlorine, magnesium, phosphorus, silicon, sodium and sulphur.
(a) In the table below, write the formulae of the hydrides formed by the elements listed. State the oxidation states (or valences) of the elements in these hydrides and classify the bonding in the hydroxides as ionic or covalent. (6marks)
| Elements | Formula of hydride | Oxidation(or valence) of the elements in these hydrides | Type of bonding |
| Aluminium | |||
| Chlorine | |||
| Magnesium | |||
| Phosporus | |||
| Silcon |
(b) The hydrides formed by sodium and sulphur were separately shaken with water.
Write the equations to show the reactions that took place, if any with; (3marks)
- sodium hydride.
- Sulphur hydride. (3marks)
Trial 6
(a) Describe how sodium hydroxide can be prepared on industrial scale (Your answer should include equations for the relevant reaction)
(b) Write equation and state the conditions under which sodium hydroxide can react with
(i) Aluminium
(ii) Phosphorous
(iii) Chlorine
Trial 7
The atomic number and melting points of oxides of elements of period III of The Periodic Table are shown in the table below
| Element | Na | Mg | Al | Si | P | S | Cl |
| Atomic number | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Oxide | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl2O7 |
| Melting point of oxide | 1275 | 2327 | 2007 | 1607 | 560 | 30 | -91 |
(a)(i) Plot a graph of melting point of oxide against atomic number. (04marks)
(ii) Explain the shape of the graph you have drawn in (a)(i) above
(b) Write equation to show the reaction between
(i) Water and P2O5 (1 ½ marks)
(ii) Sodium hydroxide and
Al2O3 (2 ½ marks)
SiO2 (2 ½ marks)
SO3 (1 ½ marks)
(iii) Hydrochloric acid and Al2O3 (1 ½ marks)
Trial 7
(a) Write an equation for the reaction between and the hydride of
Sodium
Silcon
Sulphur
(a) Write an equation for the reaction between sodium hydroxide solution
Aluminium oxide
Phosphorus pentoxide
Sulphur dioxide

Defіnitеly believe that that you stated. Your
favorite juѕtification appeared to be at the net the easіest thing to be aware of.
I say to you, I certainly get irked even as other рeople consider issues that they just ⅾon’t
recognize about. Yoս controlleԀ to hit the nail uρon the
highest and outlined out the entіre thing with no need
side effect , other folks can take a signal. Will probably be back to get more.
Thanks
Thanks for being such a source of insight. Electronics
You always make things easy to understand. chhattisgarh news
You have a real talent for this topic. Transfer Latest
Get direct access to top medical institutions through MBBS Direct Admission in Haryana.
Learn how to secure a medical career through MBBS Admission Through Management/Nri Quota in Punjab.
Join millions of players using the Raja Luck App for entertainment and rewards.
Find valuable insights on Raja Luck and make informed choices.
Discover the excitement of Goa Game, a platform that brings traditional color prediction games to your fingertips.
Learn the advantages of using an Invite Code to enhance your experience on TS EARN.
This is an excellent resource for anyone wishing to discover Why Backlinks Matter.
Handle brand-new gaming obstacles and unlock exclusive benefits on bdg win app.
Explore action-packed adventures with 55 club games and obstacle players worldwide.
Strategy your IT tasks effectively with a well-defined IT Roadmap to attain long-lasting success.
I have attempted a number of meals from Uttarakhand Foods, and each one has exceeded my expectations.
If you’re searching for ways to reduce trainee loan financial obligation, this guide on Student Loan Forgiveness Programs Everything You Need to Know is a must-read.
Anybody searching for a trusted Property Dealer Near Pari Chowk Greater Noida should visit this website.