
Chlorine and its compounds (O-level chemistry)

Halogens or Group 7B elements
These are fluorine, chlorine, bromine, iodine and astatine. Table 14.0 gives some properties of group 7B elements.
Table 14.0 Some properties of Group VIIB elements
| Element | Electron configuration | M.P. (0C) | B.P. (0C) |
| F | 2:7 | -233 | -118 |
| Cl | 2:8:7 | -103 | -34.6 |
| Br | 2: 8:18:7 | -7.2 | 58.8 |
| I | 2:8:18:18:7 | 113.5 | 184.3 |
General comment
- Halogens are diatomic nonmetals
- Melting and boiling point increase down the group due to an increase in molecular mass, chlorine is a greenish-yellow gas, bromine is a yellow volatile liquid, and iodine is a dark shiny solid.
- They react by accepting an electron to form octet configuration and they form ions of form X–
- They are the most reactive nonmetals, but the reactivity decreases down the group due to reduction of nonmetallic character
Preparation of chlorine.
Chlorine occurs as NaCl, KCl, MgCl2, and so on in seawater, salt lakes, and as deposits originating from the prehistoric evaporation of salt lakes. Chlorine is obtained by electrolysis of brine – older technology employed a mercury cathode in which the sodium is dissolved.
Na+(aq) + e– → Na(l)
2Cl– (aq) – 2e → Cl2 (g)
However, this process entailed a hazard because of the loss of mercury to the environment, and some newer process employing membrane cells and not requiring mercury is now common.
In the laboratory:
it is obtained by oxidation of concentrated hydrochloric acid with potassium permanganate (VII) or with manganese (iv) Oxide.
MnO4– (aq) + 8HCl (aq) → MnCl2 (aq) + 4H2O (l) + 3Cl2 (g)
MnO2(s) + 4HCl(aq) → MnCl2 (aq) + 2H2O (l) + Cl2
Apparatus for laboratory preparation of dry chlorine gas
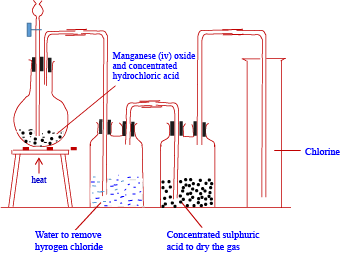
Note that chlorine is collected by downward delivery or upward displacement of air because it is denser than air
Chemical properties
- Chlorine as breaching agent
Chlorine bleaches a few drops of litmus solution dropped in a jar of chlorine. The bleaching property is due to the presence of hypochlorous acid(HOCl) from the reaction of chlorine with water.
Cl2(g) + H2O(l) → HOCl (aq) + HCl(aq)
Hypochlorous acid is very reactive compound and readily give up its oxygen to the dye, to form a colorless compound
Colored dye + HOCl → HCl + colorless dye
2. Reactions with turpentine
When a filter paper dipped in turpentine is dropped into a jar of chlorine; Chlorine and turpentine react violently with a red flash giving off a black cloud of solid particles of carbon and hydrogen chloride
C10H16 (l) + 8Cl2 (g) → 10C(s) = 16HCl (g)
3. Reaction with phosphorus
When a piece of phosphorus is dropped into a jar of chlorine, it burns spontaneously, giving off white fumes of chlorides of phosphorus, mainly PCl3.
P4(s) + 6Cl2 (g) → 4PCl3 (g) (phosphorus (III) chloride)
4. Reaction with hydrogen sulphide
When a jar of hydrogen sulphide is inverted on a jar of chlorine, yellow particles of sulphur form due to oxidation of hydrogen sulphide to sulphur by chlorine.
H2S(g) + Cl2 (g) → 2HCl (g) + S(s)
5. Reaction with hydrogen
Chlorine reacts with hydrogen to form hydrogen chloride gas
Chlorine, bromine and iodine combine with many non-metals for example.
H2(g) + Cl2 (g) → 2HCl (g)
Hydrogen chloride forms white fumes in damp air and white fumes with ammonium chloride.
NH3 (g) + HCl (g) → NH4Cl (s)
6. Reaction with metals
Chlorine reacts with many metals to form chloride, for instance, magnesium burns in chlorine to form a chloride
Mg (s) + Cl2 (g) → MgCl2(s)
Being a strong oxidizing agent it forms chloride with metal in the highest oxidation state. For instance, chlorine reacts violently with iron (III) chloride and not iron (II) chloride
2Fe(s) + 3Cl2 (g) → 2FeCl3 (s)
Apparatus for preparation Iron (III) chloride
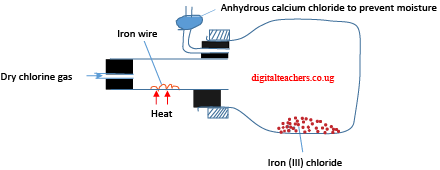
Iron (III) chloride is delinquent it absorbs moisture from the air to form a brown solution.
7. Reaction with water.
Chlorine and bromine are moderately soluble in water (bromine more so than chlorine); while iodine is only sparingly soluble. Chlorine is hydrolyzed in water to some extent.
Cl2 (g) + H2O (l) → HCl (aq) + HOCl (aq) [chloric (I) acid]
When chlorine water is exposed to sunlight, chloric acid (I) decomposes to liberate oxygen
2HOCl (aq) → 2HCl (aq) + O2 (g)
8. Reaction with iron II salts
When chlorine is bubbled through a green solution of Iron II salt solution, the color changes to yellow due to oxidation of iron II salt ions to a brown solution of Iron III salt ions.
2Fe2+ (aq) + Cl2(g) → 2Fe3+ (aq) + 2Cl–(aq)
9. Displacement of bromine and iodine from bromides and iodides.
When chlorine is bubbled into potassium bromide and potassium iodide, a yellow solution and brown solution respectively form due to oxidation of bromide and iodide ions to bromine and iodine.
2Br– (aq) + Cl2 (g) → Br2(aq) + 2Cl–(aq)
2I–(aq) + Cl2 (g) → I2 (aq) + 2Cl– (aq)
10. Reaction with alkalis.
Chlorine reacts with cold dilute sodium hydroxide solution to form pale yellow solution a chloride and sodium chlorate (I).
2OH– (aq) + Cl2 (g) → Cl– (aq) + ClO– (aq) + H2O (l)
Chlorine reacts with warm concentrated sodium hydroxide solution to give sodium chloride and sodium chlorate (V):
6OH– (aq) + 3Cl2 (g) → 5Cl– (aq) + ClO3– (aq) (chlorate V) + 3H2O (l)
Uses of chlorine
- To make bleaching agents
- Formation of polymer
- It is a disinfectant in swimming pool
Hydrogen chloride
a) Preparation
(i) Direct synthesis:
By reacting chlorine with hydrogen to form hydrogen chloride gas which dissolve in water to form the acid.
H2 (g) + Cl2 (g) → 2HCl (g)
Laboratory preparation
ii) Reaction of an ionic halide with concentrated sulphuric acid.
Sodium chloride reacts with concentrated sulphuric acid to form hydrogen chloride gas. The gas is dried by concentrated sulphuric acid and collected by downward delivery because it is denser than air.
NaCl (aq) + H2SO4 (aq) → NaHSO4 (aq) + HCl (in cold)
NaHSO4 (s) + NaCl (s) → Na2SO4 (s) + HCl (g) (on heating)
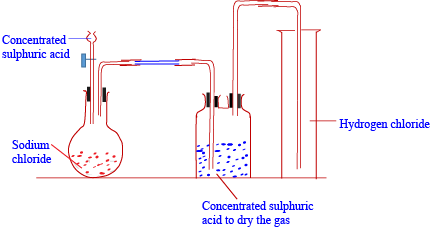
b) Physical properties of hydrogen chloride
- It has a chocking irritating smell, is an acidic gas which is very soluble in water to form hydrochloric acid.
- In water it is completely ionized to form hydrogen and chloride ions. Due to complete ionization in water, it is a strong electrolyte and conducts electricity. However, in solution with methylbenzene, it is unionized and does not conduct electricity.
- Being an acid:
- liberates hydrogen with electropositive metals such magnesium, zinc and iron.
Mg (s) + 2H+(aq) → Mg2+(aq) + H2(g)
Note that hydrochloric acid does not react with copper because copper is below hydrogen in the reactivity series.
- neutralizes bases
H+(aq) + OH–(aq) → H2O(l)
- liberates carbon dioxide from hydrogen carbonates and carbonates
H+ (aq) + HCO3– (aq) → H2O(l) + CO2 (g)
2H+(aq) + CO32– (aq) → H2O(l) + CO2(g)
– Halides
Preparation:
By reacting metals with chlorine and hydrogen chloride
For instance, when iron reacts chlorine it gives iron (III) chloride whereas hydrogen chloride gas reacts with iron to form iron (II) chloride.
2Fe (s) + 3Cl2 (g) → 2FeCl3 (s)
Fe (s) + 2HCl (g) → FeCl2 (g) + H2 (g)
For questions and answers download PDF

This is nice
It’s a very helpful sute
This is exactly what I needed to read today. Computer & Accessories
I’m excited to see what’s next! Top adult movies
Learn about the affordable MBBS Fees Structure in Andhra Pradesh for aspiring medical students.
Discover how MBBS Admission Through Management/Nri Quota in Jharkhand can make your career path smoother.
The Raja Luck App brings you thrilling games at your fingertips.