
Describe an experiment to determine the specific heat capacity of a solid using the method of mixtures.

Measurement of specific heat capacity of a solid by the method of mixtures
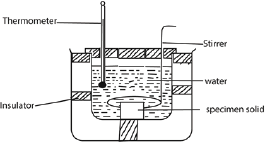
- A solid on mass, ms kg and specific heat capacity, cs, is heated in boiling water at temperature at temperature θ10C and quickly transferred to a calorimeter of heat capacity, C, containing water of mass, m1 and , at the temperature θ2.
- The final constant temperature θ3 of the mixture is determined.
Assuming there is no heat loss
Heat lost by the solid = heat gained by calorimeter + heat gained by water
ms x cs x (θ1 – θ3) = (C + m1)(θ3 – θ2)

where cw is specific heat capacity of water
Precautions taken
- The calorimeter must be heavily lagged to minimize heat loss from the mixture.
- Transfer the solid fast to minimize heat loss from it during the transfer.
- Stir the mixture gently to ensure uniform temperature distribution without causing splashing or heat loss.
- Carry out the experiment several times to minimize errors
- Make correction for heat loss.
Please obtain free notes, exams and marking guides of Physics, chemistry, biology, history, economics, geography … from digitalteachers.co.ug website.
Thanks
Dr. Bbosa Science
CATEGORIES General
TAGS Dr. Bbosa Science
