
Describe the extraction of iron from one its ore

Extraction of iron from hematite,Fe2O3
Main Impurities
Silica, SiO2
Alumina, Al2O3
Procedure
Iron ore, lime stone and coke in the correct proportions are reacted with
Preheated air in a blast furnace.
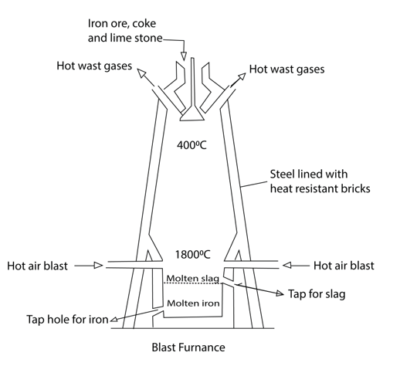
Reactions leading to reduction of iron oxide are
C (s) + O2 (g) →CO2 (g)
CO2 (s) + C (s) →2CO (g)
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
Reactions leading to removal of impurities
CaCO3 (s) → CaO (s) + CO2 (g)
CaO (s) + SiO2 (s) → CaSiO3 (l) slag
CaO (s) + Al2O3 (s) → CaSiO3 (l) slag
Molten iron and slag percolate to the bottom of the furnace and taped of through different taps.
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES Inorganic chemistry questions
TAGS Dr. Bbosa Science
