
Describe the spectra of a hydrogen atom. Use a diagrams to illustrate your answer.

Describe the spectra of a hydrogen atom. Use a diagrams to illustrate your answer.
Hydrogen produces both absorption and emission spectrum
- Absorption spectra
It is observed as dark lines on a black background when white light is passed through gaseous hydrogen. This is caused by hydrogen atoms absorbing energy corresponding to certain wavelengths from the light.
- Emission
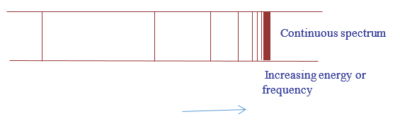
It is observed as a pink glow when an electric discharge is passed through hydrogen at low pressure. Analyzed through a spectrometer, the emission is seen to be a number of separate sets of lines or series of lines.
In each series, the intervals between the frequencies of the lines become smaller and smaller towards the high frequency end of the spectrum until the lines run together or converge to form a continuum of light or continuum.
Please Subscribe to promote this website. Subscription is free
Share with a friend
Thank you so much

I have loved da source
T has got alot of authentic information