
Describe, with the aid of a diagram an experiment to determine specific heat of vaporization of steam using the method of mixtures.

Describe, with the aid of a diagram an experiment to determine specific heat of vaporization of steam using the method of mixtures.
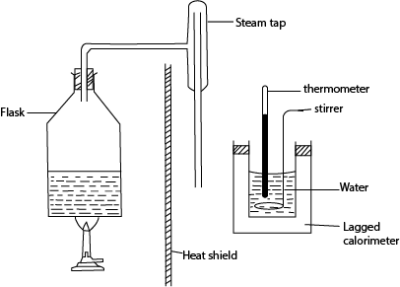
- The initial temperature θ0 and mass, m of water in the calorimeter are measured
- Steam from boiling water is passed into water in a calorimeter and after a reasonable temperature rise, flow of steam is stopped and final temperature, θf is recorded.
- Mass m2 of water in the calorimeter is then taken
- The mass of steam condensed, ms = (m2– m)
Given that the heat capacity of the calorimeter= C
Heat gained by steam = heat gained by water and calorimeter
mscv + msc(100- θf) = (m2 – m)c(θf – θ0) + C(θf – θ0)
cv = specific latent heat of vaporization
c= specific heat capacity of water
Please obtain free notes, exams and marking guides of Physics, chemistry, biology, history, from digitalteachers.co.ug website.
Thanks
Dr. Bbosa Science
CATEGORIES heat A-level
TAGS Dr. Bbosa Science

Your content is a real gem. Toys & Games
You have a real talent for this subject. 500 ka redeem code
Fast-track your admission to leading colleges via MBBS Admission Through Management/Nri Quota in Andhra Pradesh.
Learn about hassle-free enrollment at MBBS Direct Admission in Delhi.
Play your favorite games anytime with the Raja Luck App.
Find answers to key questions about Raja Luck.
Get special discounts and cashback offers when you use the 82 Lottery Invite Code throughout registration.
If you don’t know What Are Backlinks, this guide is a must-read!
Usage white-hat methods to Build backlinks for website and avoid Google charges.
Claim your free Play Store credit today with a valid 100 Google Play redeem code.
Release high-speed IT options with safe and reliable Server Rental in Chennai.