
Distribution law/partition coefficient (A -level physical chemistry)

Equilibrium between immiscible liquids
Distribution Law:
States that a solute distributes itself between immiscible solvents such that a constant temperature and pressure the ratio of its concentrations in the two solvents is constant at a given temperature
That is,
If C is the solute, and A and B are the immiscible solvents, them

Limitations
- Solute should be in the same molecular state in both solvents
- Temperature should be constant
- None of the solvent should be saturated / solutions should be dilute.
Definition
Partition Coefficient or distribution constant is ratio of the concentration of the solute in immiscible solvents at equilibrium.
Experiment to determine partition coefficient
- By finding the ration of concentrations of a solute in one solvent over the concentration a solute in another immiscible solvent at equilibrium.
- The concentration of a solute in a solvent may be obtained by titration or other reliable method.
Application of distribution laws
1. Extraction of organic compound
Example 1
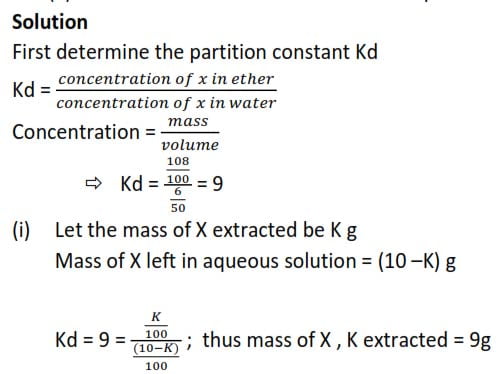
A solution of 6g of a substance X in 50cm3 of aqueous is in equilibrium at room temperature with an ethereal solution of X containing 108g of x in 100cm3.
Calculate the mass of x that could be extracted by shaking 100cm3 of an aqueous solution containing 10g of X with:
- 100cm3 of ether
- 50cm3 of ether twice at room temperature.
Solution
First determine the partition constant Kd

- Study of complexes
- The moles of a ligand that forms a complex with a metal ion = to the total moles of ligand in aqueous solution – moles of ligand that did not form a complex
- The mole of a ligand that did not a complex can be found partition coefficient, Kd
- The moles of ligand that form a complex per mole of a metal cation is obtained by division of the moles that formed a complex by the moles of metal ions
Example 2
Excess ammonia was shaken with equal volume of trichloromethane
And a 0.05M aqueous solution of copper (II) ions to form a complex (Cu(NH3)n]2+. At equilibrium, the concnentrations of ammonia in the trichloromethane and in the aqueous layer were 0.021moll-1 and 0.725moll-1 respectively.
(the partition coefficient, KD, of ammonia between water and trichloromethane is 25)
Calculate:
(i) The concentration of free ammonia in aqueous layer
(ii) The concentration of ammonia that formed the complex with copper ions
(iii) The values of n in the complex
Watch this
Revision questions? Download PDF

This is exactly what I needed to read today. Car & Motorbike
I’m always impressed by your writing. Barcelona News
Secure premium educational opportunities with MBBS Admission Through Management/Nri Quota in Rajasthan.
Discover cutoff marks for admission at MBBS Cutoff Of Private Medical Colleges in Telangana.
Join Raja Luck to participate in engaging games and exciting challenges.
Easily access your account through the Goa Games Login portal and manage your gaming activities efficiently.
Increase your referral bonuses and rewards by inviting new users with your Invitation Code.
Increase your website’s credibility with expert-approved SEO backlinks.
Get immediate cashback and unique advertising benefits with the Diu Win Invite Code.
Stay ahead in the tech market with advanced Server Rental in Noida backed by outstanding assistance.
Contend against knowledgeable players and showcase your skills on bdg-win.
Join the ultimate gaming experience with 55-club and check out awesome difficulties.
Make notified purchases with Smart Shopping Guides that deal professional recommendations.
Only MP3 is a necessary tool for music enthusiasts who wish to listen offline.
I’m preparing my trip next month and will absolutely consider Escort Service In Nainital based on these recommendations. Thanks!
Traditional Recipes like these deserve preserving and sharing.
Why choose a Chennai Escorts Agency when you can go to a luxury resort?