
Electro-chemical conductivity (A-level physical chemistry)

Electro-chemical conductivity
Resistance R of electrolytic solution
This is the drag force experienced by ions as they migrate towards the respective electrodes. Resistance of an electrolyte is proportional to the length l of an electrolyte between electrodes and inversely proportional to the square root of the cross section area A, of each electrode.

ρ is called resistivity of an electrolyte
Definition
Conductivity is the resistance of an electrolyte placed between electrodes each 1cm2 in area and 1cm apart.
In chemistry reciprocal terms are preferred.

Factors that affect the conductivity of electrolytes
1. Concentration of Ions in Solution
The higher the concentration of ions in solution, the higher its conductivity due to high number of conducting ions.
2. Weak/Strong Electrolyte
Strong electrolyte have higher conductivity than weak electrolytes because they are completely ionized in water to produce many ions
3. Temperature
The solubility of the solute increases with the increase in the temperature. Therefore, the conductivity of the electrolyte increases.
Secondly temperature increase the speed of ions in solution
Molar conductivity, Λc
This is the conductivity of a solution that contains 1 mole of an electrolyte.

where C = moles of an electrolyte in the solution between electrodes.
Example
The resistance of 0.1M KCl solution placed between electrodes each 1cm2 in area and 0.5 cm apart is 400Ω. Calculate the molar conductivity of the solution.

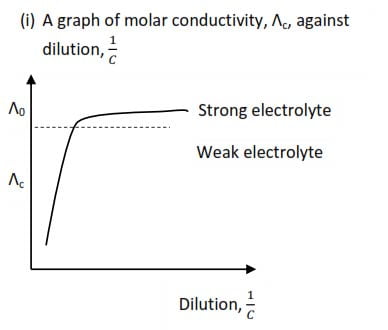
Variation of molar conductivity with concentration
Molar conductivity for both strong and weak electrolyte decrease as the concentration increase as shown by the graphs below

Explanation
(a) Strong electrolyte
- Strong electrolyte are completely ionized in solution;
- at high concentration the solution contains a high density of ions
- high density of ions lead ion interaction which reduce mobility of ions which lowers molar conductivity.
- On dilution the tendency to form these interactions reduces lowering the drag force thus molar conductivity increases.
(b) Weak electrolyte
- Weak electrolytes are poorly ionized in solution which provides low concentration conducting ions which leads to low molar conductivity compared to strong electrolytes
- At high concentration, the percentage ionization of weak electrolytes is very low leading to very few conducting ions per mole of electrolytes and low molar conductivity.
- Percentage ionization of weak electrolytes increases with dilution leading to an increase in the number of ions per mole of an electrolyte and molar conductivity.
Molar conductivity at infinite dilution, Λ0
This is the conductivity of 1 mole of electrolyte when the solution is very dilute that the ions experience no interaction from other ions.
For strong electrolyte, molar conductivity at infinite dilution is obtained by extra plotting of molar conductivity against root concentration to zero concentration.
For weak electrolyte, molar conductivity at infinite dilution is obtained by application of Kohrlrausch’s law of independent migration of ions.
It state: “the molar conductivity of an electrolyte at infinite dilution is the sum of molar conductivity of the constituent ions at infinite dilution.
For example
Λ0 NaCl = λ0 Na+ + λ0 Cl–
Λ0 AB = Λ0 AC + Λ0 BD – Λ0 CD
Example
The molar conductivity of nitric acid, potassium nitrate, and potassium fluoride are 421, 145 and 129 Ω-1cm2mol-1 respectively at infinite dilution.
Calculate the molar conductivity of hydrofluoric acid at infinite dilution.
Λ0 HF = Λ0 HNO3 + Λ0 KF – Λ0 KNO3
= 421 + 129 -145
= 405 Ω-1cm2mol-1
Factors affecting conductivity of an ion at infinite dilution
- Charge on the ion: the bigger the charge the higher the conductivity because the ion caries bigger charge per ion
- The size of an ion: small ions have high speed of movement leading to high conductivity. However, small ions with high charge have high density that may attract a big cloud of water of hydration that its effective mass may bigger than that of a big ion. That is why the small cations may have lower conductivity that big cation.
Example of conductivities of common ions at infinite dilution
| ion | λ0 (Ω-1cm2mol-1) |
| Na+ | 50.1 |
| OH– | 198.6 |
| H+ | 349.8 |
| Cl– | 76.4 |
Conductometric titrations
These are titrations in which ions are replaced by others of different conductivity, the titration followed by conductivity measurement.
(a) Titration of a strong acid with a strong base


Explanation
Initially, at A, conductivity is high due to the presence of highly conducting H+ from ionization of hydrogen chloride
HCl → H+(aq) + Cl–(aq)
Along AB conductivity decreases up to the endpoint due to the removal of highly conducting hydrogen ions.
H+(aq) + OH–(aq) → H2O (l)
Along BC after the endpoint, conductivity increases due to excess OH– ions that are relatively highly conducting.
(b) Titration of a weak acid e.g. CH3COOH with a strong base (e.g. NaOH)

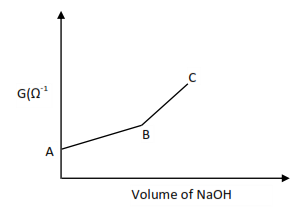
Explanation
Initially at A conductivity is low due to a small concentration of H+ ions since the acid is partially ionized.
CH3COOH ↔ CH3CO– + H+
Along AB conductivity increases up to endpoint due to the addition of salt ions and further ionization of the acid due to dilution.
CH3COOH (aq) + OH– (aq) → CH3COO–(aq) + H2O(l)
Along BC, conductivity increases due to excess OH–.
(c) Titration of a weak acid (e.g. CH3COOH) with a weak base (e.g. NH4OH)

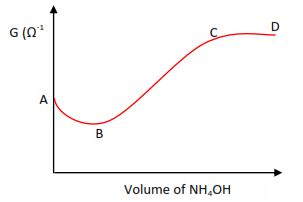
Initially, at A, conductivity is high due to presence of H+ from partial ionization of the acid
CH3COOH ↔ CH3COO– + H+
Along AB conductivity decreases due to the removal of H+.
H+(aq) + OH–(aq) → H2O (l)
Along BC conductivity increases due to addition of salt ion
CH3COOH (aq) + NH4OH (aq) → CH3COO–(aq) + NH4+(aq) + H2O(l)
Along with CD conductivity remains almost constant due to attainment of equilibrium.
Watch this
Revision question? Download PDF below
Sponsored by The Science Foundation College +256 753 80 27 09
Compiled by Dr. Bbosa Science

I’m looking forward to your next piece! Industrial & Scientific
You have a gift for making things stick. Tech News
Top MBBS Colleges in Rajasthan provide a perfect mix of theory and practical training.