
Electrolysis (O and A-level chemistry)

Electrolysis
This is the decomposition of an ionic compound in molten or solution form to its constituent elements.
Mechanism of electrolysis
Consider electrolysis of molten sodium
In molten form sodium chloride ionizes as follows
NaCl → Na+ + Cl–
In an electrolytic cell, unlike charges attract; Na+ migrates to the cathode while Cl– migrate to the anode.
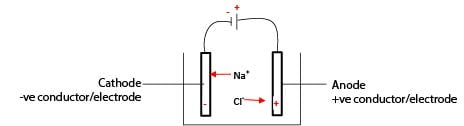
At the cathode, Na+ ion acquires an electron to become sodium atom.
Na+ + e → Na
At the anode, the Cl– loses an electron to form a Cl atom
Cl- – e → Cl
The chlorine atoms combine to form chlorine gas.
Cl + Cl → Cl2 (g)
Ultimately, sodium chloride in molten form is decomposed by electrolysis to sodium metal and chlorine gas.
Selective discharge
Consider electrolysis of sodium chloride solution
Both sodium chloride and water ionize to form ions
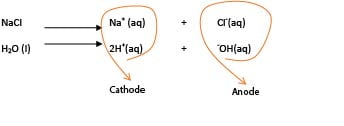
The positively charged ions migrate to the cathode while negatively charged ions migrate to the anode.
Factors that decide the ion to be eliminated or discharged first.
(a) Position in electrochemical series:
Series for cations (high to low) K+, Ca2+, Na+, Mg2+, Al3+, [C], Zn2+, Fe2+, Pb2+, Cu2+, Ag+
Series for anions (high to low) SO42-, NO3–, Cl–, Br–, I–, -OH
The ion lower in electrochemical series is discharged first. Consequently, during electrolysis of dilute sodium chloride, H+ which is low in the reactivity series than Na+ is discharged at the cathode whereas, –OH ions are discharged at the anode.
At the cathode At the anode
2H+(aq) + 2e → H2(g) 4–OH (aq) – 4e → 2H2O(l) + O2(g)
(b) Concentration:
When Cl–, Br– or I– are concentrated, then they will be discharged with respect to –OH.
In this case electrolysis of concentrated sodium chloride solution liberates chlorine at the anode and hydrogen gas at the cathode (concentration does not affect Na+)
At the cathode At the anode
2H+(aq) + 2e → H2(g) 2Cl– (aq) – 2e → Cl2(g)
(c) Nature of electrode
When mercury cathode is used Na+ is discharged with preference to H+.
Therefore, electrolysis concentrated sodium chloride using mercury cathode liberate Na at the cathode and chlorine gas at the anode (due to high concentration of Cl–).
At the cathode At the anode
Na+(aq) + e → Na 2Cl– (aq) – 2e → Cl2(g)
Application of electrolysis
- Industrial preparation of gases; Cl2, H2 and O2.
- Extraction of metal: metals above carbon in the reactivity series are almost all extracted by electrolysis.
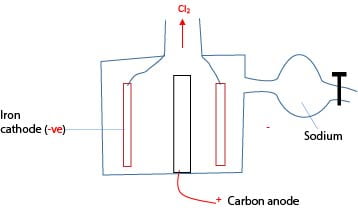
Example Extraction of sodium.
Ore: molten NaCl
Anode: the iron cylinder is cheap and has a melting point above the melting point of NaCl.
Cathode: carbon because it does not react with chloride.
Calcium chloride added to the mixture to
- lower the melting point of sodium chloride from 8000Cto 6000C,
- reduce solubility of sodium in molten sodium chloride,
- Lower the corrosive vapor of sodium chloride.
Sodium is collected in dry nitrogen to protecting it from reacting with air.
At the cathode At the anode
Na+ + e → Na 2Cl–– 2e → Cl2(g)
Setup
The setup is such chlorine produced does not react with sodium
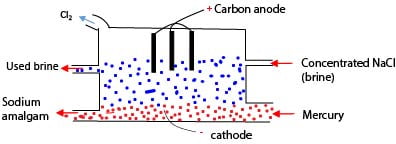
3. Preparation of sodium hydroxide
By electrolysis of a concentrated solution of sodium chloride using carbon anode and mercury cathode. At the anode chlorine is liberated and at mercury cathode Na+ instead of H+ is discharged and dissolved in mercury to form mercury amalgam.
At the cathode At the anode
Na+ + e → Na 2Cl–– 2e → Cl2(g)
When sodium amalgam is dropped in water, sodium reacts to form sodium hydroxide and hydrogen gas.
2Na + 2H2O (l) →2NaOH (aq) + H2(g)
Sodium hydroxide is concentrated to form pellets.
This process is disadvantageous because it releases poisonous mercury into the environment
Setup
4. Purification of copper
Anode: impure copper (dissolves)
Cu (s) – 2e → Cu2+ (aq)
Cathode (copper is deposited)
Cu2+ (aq) + 2e → Cu (s)
Electrolyte: copper sulphate solution
Copper migrated from the anode to the cathode.
Faraday’s Laws of electrolysis
- The mass of a substance liberated at an electrode is proportional to the quantity of electricity used.
The quantity of electricity Q in coulombs = It
(I = current of electricity in amperes, t = time in second)
2. The moles of electricity required to liberate one mole of an element is proportional to the charge on its ions. (1 mole of electricity = 1 Faraday = 1F = 96500 C)
Example
Calculate the mass of copper liberated by a current of 1A for 1hour. (Cu = 63.5)
Quantity of electricity Q= It = 1 x (1 x 60 x 60) = 3600C
Cu (s) – 2e → Cu2+ (aq)
It implies that (96500 x2) C is required to liberate 63.5 g of copper

Therefore, 3600C will liberate = 1.18g
Watch this
Revision question? Download PDF below
Sponsored by The Science Foundation college +256 753 80 27 09
Compiled by Dr. Bbosa Science

Excellent work, i would like to get more lectures downloaded for my sister who is in A’level
That is very fascinating, You are an excessively skilled blogger I’ve joined your rss feed and stay up for looking for more of your excellent post Additionally, I have shared your site in my social networks!
Hello! Quick questipn that’s entirely offf topic. Do you knbow howw to
make youjr ssite mobipe friendly? My weblog loks wedird when browsing ffrom my iphone.
I’m trying to find a template oor lugin thwt might be abvle to fixx this problem.
If you havge any suggestions, please share. Maany thanks!
I’m constantly learning from your posts. Clothes & Accessories
I appreciate the time you put into this. 500 ka redeem code
Begin your medical journey with MBBS Admission Through Management/Nri Quota in Orissa.
Discover cutoff trends at MBBS Cutoff Of Private Medical Colleges in Madhya Pradesh.
Enhance your gaming skills by playing on Raja Luck.
Improve your gaming journey with personalized deals through the 82 Lottery Invite Code.