
Electrolysis (O-level chemistry)

Electrolysis
This is the decomposition of an ionic compound in molten or solution form into its constituent elements by electricity.
Note that
- An electrolyte is an ionic substance that conducts electricity in a solid or molten form.
- Solid electrolytes do not conduct electricity because the ions cannot move because they are held together by strong ionic bonds.
Mechanism of electrolysis
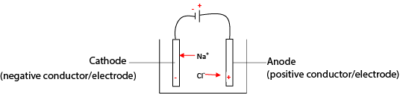
Consider electrolysis of molten sodium
In molten form sodium chloride ionizes as follows
NaCl → Na+ + Cl–
In an electrolytic cell, unlike charges attract; Na+ migrates to the cathode while Cl– migrates to the anode.
Na+ + e → Na
At the anode the Cl– loses an electron to form a Cl atom
Cl- – e → Cl
The chlorine atoms combine to form chlorine gas.
Cl + Cl → Cl2 (g)
Ultimately, sodium chloride in molten is decomposed by electrolysis to sodium metal and chlorine gas.
Selective discharge
Consider electrolysis of sodium chloride solution
Both sodium chloride and water ionize to form ions
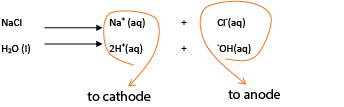
The positively charged ions migrate to the cathode while negatively charged ions migrate to the anode.
Factors that decide the ion to be eliminated or discharged first.
1. Position in electrochemical series:
Series for cations (high to low) K+, Ca2+, Na+, Mg2+, Al3+, [C], Zn2+, Fe2+, Pb2+, Cu2+, Ag+
Series for anions (high to low) SO42-, NO3–, Cl–, Br–, I–, -OH
The ion lower in electrochemical series is discharged first. Consequently, during electrolysis of dilute sodium chloride, H+ which is low in the reactivity series than Na+ is discharged at the cathode whereas, –OH ions are discharged at the anode.
At the cathode At the anode
2H+(aq) + 2e → H2(g) 4–OH (aq) – 4e → 2H2O(l) + O2(g)
2. Concentration:
When Cl–, Br– or I– are concentrated, then they will be discharged with respect to –OH.
In this case electrolysis of concentrated sodium chloride solution liberates chlorine at the anode and hydrogen gas at the cathode (concentration does not affect Na+)
At the cathode At the anode
2H+(aq) + 2e → H2(g) 2Cl– (aq) – 2e → Cl2(g)
3. Nature of electrode
When mercury cathode is used Na+ is discharged with preference to H+
Therefore, electrolysis concentrated sodium chloride using mercury cathode liberate Na at the cathode and chlorine gas at the anode (due to high concentration of Cl–).
At the cathode At the anode
Na+(aq) + e → Na 2Cl– (aq) – 2e → Cl2(g)
Application of electrolysis
- Industrial preparation of gases; Cl2, H2 and O2.
- Extraction of metal: metals above carbon in the reactivity series are almost all extracted by electrolysis.
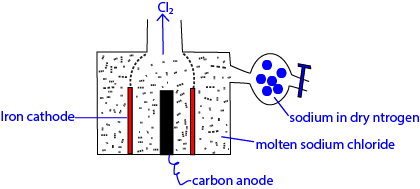
Example Extraction of sodium.
Ore: molten NaCl
Anode: iron cylinder is cheap and has a melting point above the melting point of NaCl.
Cathode: carbon because it does not react with chloride.
Calcium chloride added to the mixture to
- lower the melting point of sodium chloride from 8000Cto 6000C,
- reduce solubility of sodium in molten sodium chloride,
- Lower the corrosive vapor of sodium chloride.
Sodium is collected in dry nitrogen to protecting it from reacting with air.
At the cathode At the anode
Na+ + e → Na 2Cl–– 2e → Cl2(g)
The setup is such chlorine produced in a different compartment not react with sodium
When sodium amalgam is dropped in water, sodium reacts to form sodium hydroxide and hydrogen gas.
2Na + 2H2O (l) → 2NaOH (aq) + H2(g)
Sodium hydroxide is concentrated to form pellets.
3. Preparation of sodium hydroxide
By electrolysis of a concentrated solution of sodium chloride using carbon anode and mercury cathode. At the anode chlorine is liberated and at mercury cathode Na+ instead of H+ is discharged and dissolved in mercury to form mercury amalgam.
At the cathode At the anode
Na+ + e → Na 2Cl–– 2e → Cl2(g)
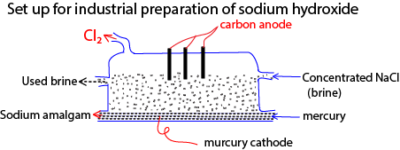
This process is disadvantageous because it releases poisonous mercury into the environment
4. Purification of copper
Anode: impure copper (dissolves)
Cu (s) – 2e → Cu2+ (aq)
Cathode (copper is deposited)
Cu2+ (aq) + 2e → Cu (s)
Electrolyte: copper sulphate solution
Copper migrated from the anode to the cathode.
Faraday’s Laws of electrolysis
- The mass of a substance liberated at an electrode is proportional to the quantity of electricity used.
The quantity of electricity Q in coulombs = It
(I = current of electricity in amperes, t = time in second)
- The moles of electricity required to liberate one mole of an element is proportion to the charge on its ions. (1mol of electricity = 1Faraday = 1F = 96500 C)
Example
Calculate the mass of copper liberated by a current of 1A for 1hour. (Cu = 63.5)
Quantity of electricity Q= It = 1 x (1 x 60 x 60) = 3600C
Cu (s) – 2e → Cu2+ (aq)
It implies that (96500 x2) C is required to liberate 63.5 g of copper
Therefore, 3600C will liberate = 1.18g
Daniels’ or electromotive cell
This is a cell that produces an electric current
It consists of two metal rods connect to each other via a voltmeter and each dipped in a solution of respective metal ions. The solution separated from each other by a porous wall or a salt bridge.
Note that
- The metal dipped into a solution of its metal ions constitute a half cell.
- The half-cell for the most reactive element is the place to the left of that of the least reactive element.
- The half-cell on the left constitutes the anode where oxidation occurs while that on the right is the cathode where reduction occurs
- In external wire, electrons move from the anode to the cathode while current move in the opposite direction from the cathode to the anode
- The porous partition or salt bridge serves to complete the circuit.
An example of an electromotive cell made of copper and zinc is shown below
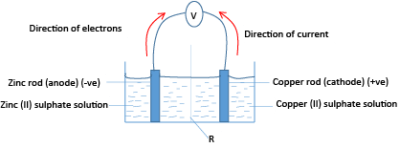
The cell consists of a zinc rod dipped in zinc sulphate and a copper rod dipped in copper sulphate solution, the solutions separated by a porous wall, and the rods connect by wires to a voltmeter.
Zinc half-cell a constitutes the anode while copper half-cell constitutes the cathode
Reaction equation on zinc electrode (anode): oxidation occurs
Zn – 2e → Zn2+(aq)
Reaction equation on the Copper electrode (cathode): reduction occurs
Cu2+(aq) + 2e → Cu(s)
Overall equation
Zn (s) + Cu2+(aq) → Zn2+ (aq) + Cu(s)
In otherwise, the electrons from the ionization of zinc reduce copper ions to copper.
Cell nation: Zn(s)/Zn2+(aq) // Cu2+(aq) /cu
Differences between an electrolytic cell and emf cell
| Electrolytic cell | Emf/Daniel’s cell |
| Use emf | Generate emf |
| Use single electrolytes | Uses two electrolytes |
| An anode is a positive electrode | An anode is a negative electrode |
| The cathode is the negative electrode | The cathode is the positive electrode |
For revision question and answers download

I need mathematics and physics notes for both O and A level.
good notes sir
Very helpful information, thanks
Very good answers
nice work requesting for tests
I can’t wait to share this with my friends. Baby Products
I’m looking forward to your next piece! 500 ka redeem code