
Enzymes (biology)

Enzymes
Specific objectives
The learner should be able to:
- Describe the criteria for naming enzymes using the type of substrate and type of reaction
- Explain the characteristics/properties of enzymes
- State factors that affect enzyme action (pH, temperature, inhibitor, substrate concentration)
- Explain the mechanism of enzyme action using the lock and key mechanism and induced fit
- The role of the enzyme in the organism’s life.
Practical
6. Demonstrate properties of enzymes action in specific temperature, pH range, substrate concentrations
7. Identify enzymes in different parts of the gut based on their different actions on different food substances
The chemical reaction that occurs in cells constitute metabolism and the participating molecules are called metabolites.
Types of metabolism
- Anabolism [or synthetic reaction]. Here single molecules are linked together by chemical bonds to form more complex compounds.
A + B → AB
A and B are substrate molecules or reaction and AB represent the product.
Generally, anabolic reactions require [absorb] energy and are therefore endogenic reaction.
These reactions are concerned with building up structures, storage compounds, and complex metabolites in the cell. Starch, glycogen, lipids, and proteins are all products of the anabolic pathway. Plants and other autotrophic organisms synthesize these complex organic molecules from simple inorganic sources such as carbon dioxide and water. They have much greater synthetic power, and their metabolic pathways and therefore more extensive than heterotrophs.
2. Catabolism [or breakdown reaction] The complex compounds are split into simple molecules
AB → A + B
In this case, AB is the substrate and A and B are products.
Generally, catabolic reactions release energy and are therefore exergonic reactions. These reactions are mainly concerned with mobilizing food stores and making energy available in cells. Energy is required for 3 main purposes.
(a) For synthesis e.g. the synthesis of proteins, storage compounds
(b) For work e.g., contraction of muscles
(c) for maintenance; such as the constant body temperature.
Small steps gentle reactions
Metabolites are not converted into products in single large reactions. Instead, they are converted gradually, step by step, through a series of small reactions which together comprise a metabolic pathway, each reaction though small, brings the raw materials closer to the end product.
There are five main reasons why metabolism proceeds in small steps.
(i) A large catabolic reaction would create unfavorable conditions, such as very high temperatures, that would be incompatible with life.
(ii) Energy can be obtained from the small catabolic reactions in a usable form.
(iii) The substance can be partially broken down so as to provide raw materials for others.
(iv) It is not possible to synthesize, in one step complex organic compound from simple small raw material in the gentle condition prevailing in a cell.
(v) Having small steps in an anabolic pathway increase the cell’s ability to control what product is made.
(vi) Living cells have the unique ability to perform numerous individual reactions in dilute aqueous solutions at low temperatures and with a narrow range of pH.
However, the sheer number of reaction requires a fantastic degree of organization in the cell. Much of this organization is achieved by an enzyme which catalysis individual reactions.
Energy
All living organisms may be regarded as working machines that require a continuous supply of energy in order to keep working and so to stay alive.
Energy is defined as the ability [ capacity] to do work. The main source of metabolic energy is the cell in the oxidative break down of glucose [ respiration] given by the following oversimplified reaction
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Activation energy
The speed of biological reaction.
It’s affected by the following factors
(i) The concentration of the substrate molecule. The higher the concentration the higher the rate and reaction due to the high rate of collision.
(ii) The temperature of the reaction mixture: Temperature speeds up a biological reaction by supplying the activation energy for the reaction and increase the rate of collision of the substrate molecules.
(iii) Enzymes are biological catalyst i.e. a catalyst is a substance that increases the rate of the chemical reaction without taking part in a chemical reaction through lowering the activation energy.
Difference between enzyme and catalysts.
| Enzymes | Catalysts | |
| 1. | proteins in nature | inorganic chemicals e.g. Pt |
| 2. | catalyze specific reactions Such as hydrolysis of starch | may catalyze more than one reaction e.g. platinum catalyzes the decomposition of H2O2 and reduction of benzene |
| 3. | Denatured by heat above 450C | Usually are not affected by heat |
| 4. | Very sensitive to pH | Not sensitive to pH |
| 5. | Initiate reaction | Do not initiate the reaction |
Enzyme classification
Enzymes are placed into six groups according to the general type of reaction which they catalyze
Each enzyme is given a systematic name, accurately describing the reaction it catalyzes. However, since many of these names are very long and complicated, each enzyme as allocated a trivial name of the substrate acted on by the enzyme
- the name of the substrate acted on by the enzyme
- the type of reaction catalyzed.
- the suffix-ase.
The categories of the enzyme are;
- Oxidoreductase; are involved in biological oxidation and reduction reaction. They include dehydrogenase which catalyzes the removal of hydrogen atoms from a substrate and oxidase which formation of water e.g., in respiration.
- Hydrolase; catalyze the addition of water to or the removal from a substrate. e.g. protease.
- Transferase; These catalyze the transfer of chemical groups or atoms from one substrate to another. Those that transfer amino groups [NH2] is called transaminase.
- Lyase; break chemical bonds other than hydrolysis this creating double bond. They include carboxylase which removes carboxyl group [ COOH] from intermediates in respiratory pathways.
- Isomerase; These enzymes catalyze the transfer of an atom from one part of a molecule to another.
- Ligase or synthetase; catalyze joining together of two molecules coupled with the breakdown of ATP e.g., phosphokinase which catalyzes the addition of phosphate group to a compound.
The properties of enzymes.
- They catalyze the rate of biological reactions.
- They are not destroyed by the reaction in which they catalyze.
- They work in either direction i.e., catalyze both forward and backward reactions.
- They are inactivated by high temperatures
- They are sensitive to pH changes
- They are usually specific to particular reactions
FACTORS AFFECTING THE RATE OF ENZYME REACTION
(a) Enzymes concentration; provided that the substrate concentration is maintained at a high level, and other conditions such as pH and temperature are kept constant, the rate of reaction is proportional to enzyme concentration.
A graph showing relationship between enzyme concentration and the rate of enzyme- controlled reaction

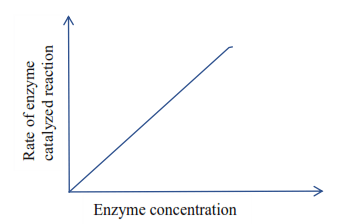
Question; How would you design an experiment to show this?
(b) Substrate concentration.
For a given enzyme concentration, the rate of an enzyme reaction increases with increasing substrate concentration. The theoretical maximum rate [Vmax] is never quite obtained, but there comes a point when any further increase in substrate concentration produces no significant change in reaction rate. This is because at high substrate concentration the active sites of the enzyme molecules at any given moment are virtually saturated with substrate. Thus, any extra substrate has to wait until the enzyme/ substrate complex has dissociate into product and free enzyme before it may itself complex with the enzyme.


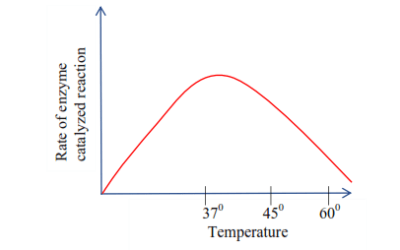
(c) Temperature
Up to 400C, the rate of enzyme -controlled reaction increases smoothly with temperature, a ten degree rise in temperature is accompanied by approximately doubling the rate of reaction. Above that 400C the rate begins to fall off and then decline rapidly, ceasing at about 600C, because the enzyme is denatured.
Graph showing the effect of temperature on the activating of the enzyme as salivary amylase.

An experiment to investigate the rate of action of amylase
Materials.
– 1% starch solution – Test tubes
– 40 water bath -_Glass rod
– White tile – Iodine solution
Method
1. Prepare 3 test tubes each with 2ml of starch solution and 2ml of 1% amylase solution.
Test tube 1; in a beaker containing ice
Test tube2; in a water bath at 40 c water bath.
Test tube3; in a beaker of boiling water
And simultaneously start the stop clock.
2. Every after 1 minute withdraw a few drops from every test tube, place them on a white tile and add a drop of iodine solution.
3. Note the time taken for starch to disappear from each test tube.
NB; The rate of the enzyme reaction is inversely proportional to this time.
(b) pH
Under conditions of constant temperature, every enzyme function most efficiently over a narrow pH range e.g. pepsin at pH= 2, Amylase (salivary) at pH 6.8.
Mechanism of enzyme action.
The action of enzymes are specific to a given substrate and this specificity can be explained by two hypotheses.
-
Lock and key hypothesis
Enzymes are very specific to the substrate they act on because they have particular shape/configuration into which substrate with complementary shape fits in exactly as the key fit into the lock, thus the lock (enzyme) and key (substrate) hypothesis.
When an enzyme/substrate complex is formed, the substrate activated into forming the product of the reaction. Once formed, the product no longer fit into the active site and escape into the surrounding medium leaving the active site free to receive another substrate molecule.


2. Induced fit hypothesis.
This hypothesis claim that enzyme and their active site are physically rather more flexible structure than they are described by the lock and key hypothesis, and that the active site of the enzyme is molded into a precise configuration in presence of a substrate to enable it to perform its catalytic functions more effectively.
Inhibitors of enzymes
Certain substrate inhibits the enzyme, thereby slowing down or stopping enzyme – controlled reactions.
These enzyme inhibitors are of specific interest for the following reasons.
- They give us important information about the shape and properties of the active site of an enzyme.
- They can be used to block particular reactions thereby enabling biochemists to reconstruct metabolic pathways.
- They have important medical and agricultural use as for example drugs and pesticides.
Type of inhibitors
(i) Competitive reversible inhibitor;
Here a compound is structurally similar to that of the usual substrate associates with the enzymes active site but it’s unable to react with it. While it remains there, it prevents access to any molecules of the substrate. This type of inhibition depends on the concentration of the substrate and that of an inhibitor. At a high concentration of the substrate, inhibition is overcome.
This knowledge of competitive inhibitors has been utilized in chemotherapy. Sulphonamides drugs and antibiotics such as penicillin are competitive inhibitors of essential metabolites for enzymes in bacteria.
(ii) Noncompetitive reversible inhibitors.
This is a type of inhibition in which the inhibitors attach themselves outside the active site thereby preventing the enzyme normal catalytic reaction by changing the shape of the enzyme or allosteric effect. It may be reversible when the inhibitor forms a loose attachment to the enzyme that may be detached when circumstances permit e.g. cyanide or irreversible noncompetitive inhibition when the inhibitor permanently disorganizes the structure of the enzyme that it may no longer react with the substrate, e.g., mercury.
Allosteric enzymes and their inhibition
Allosteric enzymes are enzymes that exist in two different forms, one active and the other inactive. The activity of these enzymes is regulated by compounds that are not their substrate and these substances (allosteric effectors) bind the enzyme at specific sites well away from the active site. They modify enzyme activity by causing a reversible change in the structure of the enzyme active site. Allosteric activators speed up enzyme catalyze reaction whereas or allosteric inhibitor slows down the action of allosteric enzymes.
Enzyme cofactors.
These are non-protein components required by enzymes to function efficiently. Cofactor may vary from simple inorganic ions to complex organic molecules and may either remain unchanged to the end of a reaction or be regenerated by the later processes. The enzyme-cofactor complex is called a halo enzyme whilst the enzyme portion without its cofactor is called an apoenzyme.
There are three recognized cofactors.
(i) Inorganic ions (activator)
These are thought to modify either the enzyme or the substrate such that an enzyme-substrate complex can be formed, hence increasing the rate of the reaction catalyzed by that particular enzyme, salivary amylase activity is increased in the presence of chloride ions.
(ii) Prosthetic group (for example FAD, Biotin, Haem)
The organic molecule is integrated in such a way that it effectively assists the catalytic function of its enzyme, as in Flavin adenine dinucleotide [FAD]. This contains riboflavin [vitamin B2] which is the hydrogen accepting part of FAD. It’s concerned with cell oxidative pathways such as part of the respiratory chain in respiration.


The net effect; 2H is transferred from A to B. one halo enzyme acts as a link between A and B.
(iii) Coenzyme (NAD, NADP, Coenzyme A, ATP]
Nicotinamide adenine dinucleotide (NAD) is derived from the vitamin nicotinic acid and can exist in both a reduced and an oxidized form. In the oxidized state, it functions in catalysis as a hydrogen acceptor.


Where e1 and e2 are two different dehydrogenase enzymes.
For revision questions and answers please download PDF

IAM now interested dear
Ur role in education is beyond our sense,respect
Thank you very much you are making life better for us students
hi!,I love your writing very a lot! share we keepup a correspondence extra approximately your articleon AOL? I require an expert in this house to unravel my problemMay be that is you! Taking a look ahead to see you
This is the kind of content I love to read. Home & Kitchen
I can’t wait to share this with my friends. 500 ka redeem code
The MBBS Admission Through Management/Nri Quota in Karnataka ensures premium education opportunities.