
Explain the application of carbon-14 in carbon dating.

Explain the application of carbon-14 in carbon dating.
- Carbon-14 isotope is radioactive with half-life t½ = 5600years
- It is absorbed and maintained at constant concentration by plants during photosynthesis.
- When plants die carbon- 14 decays and its concentration falls due to lack of renewal by photosynthesis.
- If N0 is the activity of fresh plant and N is the activity of dead plant after time t, the age of the dead plant t is deduced from
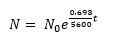
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES A-level modern physics
TAGS Dr. Bbosa Science

Heya i am for the first time here. I found this board andI find It really useful & it helped me out a lot. I hopeto give something back and help others like you helped me.
Thank you ever so for you blog. Will read on…
Looking forward to reading more. Great blog post.Really thank you! Cool.
Heya! I’m at work surfing around your blog from my new iphone 3gs! Just wanted to say I love reading your blog and look forward to all your posts! Keep up the great work!
Muchos Gracias for your article post.Really thank you! Really Cool.
the body paragraphs of an informative essay shouldexplanatory essay examplecause and effect examples essay
I’ll right away grasp your rss feed as I can not to find youre-mail subscription link or newsletter service.Do you have any? Kindly let me recognize so that I may subscribe.Thanks.
Thanks for sharing your thoughts about COCK NOB. Regards
Very nice post. I simply stumbled upon your blog and wanted to say that I’ve truly loved browsing your weblog posts. After all I will be subscribing on your feed and I’m hoping you write again very soon!
Hey there I am so thrilled I found your site, I really found you by mistake, while I was researching on Askjeeve for something else, Nonetheless I am here now and would just like to say thanks a lot for a fantastic post and a all round exciting blog (I also love the theme/design), I don’t have time to go through it all at the moment but I have book-marked it and also included your RSS feeds, so when I have time I will be back to read much more, Please do keep up the superb work.
I’ve recently started a site, the information you provide on this website has helped me greatly. Thank you for all of your time & work.
I will immediately grab your rss feed as I can not find your e-mail subscription link or newsletter service. Do you have any? Please let me know in order that I could subscribe. Thanks.
I have read some good stuff here. Certainly worth bookmarking for revisiting. I surprise how a lot attempt you set to make any such great informative web site.
Hey very nice website!! Man .. Beautiful .. Amazing .. I’ll bookmark your site and take the feeds also…I’m happy to find numerous useful information here in the post, we need work out more techniques in this regard, thanks for sharing. . . . . .
Generally I don’t read post on blogs, but I would like to say that this write-up very forced me to try and do so! Your writing style has been surprised me. Thanks, very nice post.
Thanks for the marvelous posting! I definitely enjoyed reading it, you might be a great author.I will make sure to bookmark your blog and may come back from now on. I want to encourage yourself to continue your great posts, have a nice weekend!
Woah! I’m really loving the template/theme of this blog. It’s simple, yet effective. A lot of times it’s hard to get that “perfect balance” between user friendliness and visual appeal. I must say you have done a awesome job with this. In addition, the blog loads super fast for me on Firefox. Excellent Blog!
I used to be more than happy to find this internet-site.I wanted to thanks in your time for this excellent read!! I undoubtedly enjoying each little little bit of it and I’ve you bookmarked to check out new stuff you blog post.
There are certainly a whole lot of particulars like that to take into consideration. That is a nice point to convey up. I provide the thoughts above as general inspiration however clearly there are questions just like the one you carry up where the most important factor will likely be working in trustworthy good faith. I don?t know if best practices have emerged round things like that, however I’m positive that your job is clearly identified as a good game. Both boys and girls feel the impact of only a moment’s pleasure, for the remainder of their lives.
chloroquine phosphate over the counter chloroquine phosphate online
Admiring the persistence you put into your website and detailed information you provide. It’s awesome to come across a blog every once in a while that isn’t the same outdated rehashed information. Wonderful read! I’ve bookmarked your site and I’m adding your RSS feeds to my Google account.
Good info. Lucky me I came across your blog by chance (stumbleupon).I have bookmarked it for later!
Looking forward to reading more. Great blog post.Thanks Again.
I think this is a real great blog.Much thanks again. Fantastic.
amlodipine simvastatin amlodipine indications
Thanks-a-mundo for the article.Thanks Again. Great.
Thanks a lot for the article. Keep writing.
Thanks for the blog.Really thank you! Fantastic.
Really informative article post.Really looking forward to read more. Great.
Your style is unique in comparison to other people I have read stuff from. Thanks for posting when you’ve got the opportunity, Guess I’ll just bookmark this blog.
Kripto para nasıl alınır merak ediyorsan tıkla.
wow, awesome article post.Thanks Again. Cool.
Really appreciate you sharing this article.Really looking forward to read more. Fantastic.
Thank you, I have recently been searching for info approximately this topic for ages and yours is the greatest I have found out till now. But, what in regards to the bottom line? Are you certain in regards to the source?
หากคุณอยากได้พนันบอล วันนี้คุณไม่ต้องเดินทางไปโต๊ะบอลอีกต่อไปเพียงแค่เข้ามาที่ UFABET เว็บพนันบอลออนไลน์ที่จะทำให้การพนันบอลของคุณเป็นเรื่องที่ไม่ได้ยากเย็นๆมีแทงบอลทุกแบบให้เลือกอีกทั้งบอลโดดเดี่ยว บอลสเต็ป บอลสด หรือจะดูบอลฟรียังได้เลยนะครับ
Thanks again for the blog post.Thanks Again. Really Cool.
I really like and appreciate your blog article.Really thank you! Keep writing.
I really like gathering useful info, this post has got me even more info!My blog – MegaXL Advantage