Group 3 elements (boron and aluminium) (A-level inorganic chemistry)

Group 3 elements
Members
Elements symbol
Boron B
Aluminium Al
Gallium Ga
Indium In
Thallium Tl
Boron
Boron is a nonmetal, aluminium is metal whereas gallium, Indium and Thallium are weakly metallic.
Uses of boron
1. Boron is used in making heat resistant glasses
2. Boron is an essential nutrient.
3. Sodium tetraborate (borax), Na2B4O7. 10H2O is used to standardize hydrochloric acid solutions.
Na2B4O7 (aq) + 2HCl (aq) + 5H2O (l) → 2NaCl (aq) + 4H3BO3 (s)
Aluminium
Aluminium is a light metal that strong, malleable and ductile.
Its surface is protected by a thin layer of oxides the prevents from being very reactive.
It is used for electric cables, saucepans, tins, aeroplanes.
Extraction of Aluminium
Ore: Bauxite (Al2O3.xH2O)
Major impurities are
- Silica or SiO2
- Iron salts
Principles in extraction
Extraction of aluminium involves removal of impurities (purification) and then reduction to metal by electrolysis.
Steps in the extraction of Aluminium
- The ore is heated
- to remove water and,
- To convert iron salts to iron III oxide
2. The powdered ore is heated with concentrated sodium hydroxide to dissolve aluminium oxide and silica such that the insoluble iron oxide is filtered off.
Aluminium oxide form aluminate
Al2O3 (s) + 2NaOH (aq) + 7H2O (l) → 2Na[Al(OH)4(H2O)2] (aq)
Or the ionic form
Al2O3 (s) + OH–(aq) → 2AlO2–(aq) + H2O(l)
Silica also dissolves forming sodium silicate.
SiO2 (s) + 2NaOH (aq) → Na2SiO3(aq) + H2O (l)
3. To the filtrate, a little aluminium hydroxide is added to precipitate Aluminium hydroxide,(seeding).
NaAlO2(aq) + 2H2O(l) → NaOH(aq) + Al(OH)3(s)
Alternatively, carbon dioxide bubbled through the filtrate to precipitate aluminium hydroxide as follows
2NaAl(OH)4(aq) + CO2(g) → 2Al(OH)3(s) + Na2CO3(aq) + H2O(l)
4. The precipitated aluminium hydroxide is filtered off, washed and ignited to give pure aluminium oxide (alumina).
2Al(OH)3(s) → Al2O3(s) + 3H2O (g)
5. Aluminium is obtained from aluminium oxide by electrolysis.
Cryolite, Na3AlF6, is added to
(i) lower the melting point of alumina from20500C to 9000C
(ii) and improve the conductivity of the aluminium oxide
At the cathode (carbon) aluminium is liberated
Al3+ (aq) + 3e– → Al (s)
At the anode (carbon) oxygen is liberated
2O2- – 4e → O2 (g)
The anode is eaten up by oxygen
C + O2 (g) → CO2 (g)
Trial 1
(a) During the extraction of aluminium, the ore is first purified.
(i) Write the name and formula of one ore from which aluminium is extracted. (1 marks)
(ii) Name two main impurities in the ore. (1 marks)
(iii) Name the reagent that is used in the purification of the ore. (1 marks)
(b) The purified ore is mixed with cryolite, melted and electrolyzed.
(i) State the purpose of adding cryolite. (1 mark)
(ii) Name the electrodes used. (1 mark)
(iii) Write an equation for the reaction that takes place at the cathode during the electrolysis. (2 marks)
(c) Write an equation to show how anhydrous aluminium chloride can be obtained from aluminium. (2 marks)
Trial 2
(a) Write the formula of ore of aluminium.
(b) During the extraction of aluminium, the ore is first treated with sodium hydroxide, followed by aluminium hydroxide.
(i) State the purpose of adding sodium hydroxide. (1.mark)
(ii) Write an equation for the reaction between the ore and sodium hydroxide. (1½ marks)
(iii) What is the purpose of adding aluminium hydroxide? (1 mark)
(b) Briefly explain how aluminium can be obtained after the ore has been treated as in (b). (3.marks)
(c) Carbon dioxide was used instead of aluminium hydroxide in (b). Write an equation for the reaction that took place.
Reactions of aluminium
(a) with non-metals
Aluminium combines directly with oxygen, sulphur, nitrogen and halogens at appropriate conditions.
4Al (s) + 3O2 (g) → 2Al2O3(s)
4Al (s) + 6S (s) → 2Al2S3(s)
2Al (s) + N2 (g) → 2AlN (s)
2Al (s) + 3F2 (g) → 2AlF3(s)
The oxide and fluoride are ionic, and the rest are predominantly covalent.
(b) Reaction with HCl
Aluminium reacts with moderately concentrated HCl to give a chloride and hydrogen gas.
2Al (s) + 6HCl (aq) → 2AlCl3(s) + 3H2(g)
(c) with sulphuric acid
Aluminum is not readily attacked by dilute sulphuric acid, but with the concentrated acid it gives the sulphate and sulphur dioxide and water.
2Al (s) + 4H2SO4(aq) → Al2(SO4)3(s)+ 2H2O(l) + SO2(g) + 2H2(g)
(d) Reaction with nitric acid
Aluminium does not react with nitric acid probably due to formation of an impenetrable oxide layer on the surface.
(e) Reaction with sodium hydroxide
Aluminium reacts with sodium hydroxide liberating hydrogen gas.
2Al(s) + 2–OH(aq) + 6H2O(l) → 2Al(OH)4–(aq) + 3H2(g)
Halides of aluminium
(a) Aluminium fluoride (AlF3)
It can be made by reacting metallic aluminium with fluorine. It is the only ionic aluminium halide and it is sparingly soluble in water.
(b) Aluminium chloride, Al2Cl6
Preparation:
1. By passing hydrogen chloride or chlorine overheated metal under anhydrous conditions.
2Al(s) + 3Cl2 (g) → Al2Cl6 (s)
2Al(s) + 6HCl (g) → Al2Cl6 (s) + 3H2 (g)
2. By passing chlorine gas over a mixture of aluminium oxide and charcoal heated to about 10000C.
Al2O3 (s) + 3C (s) + 3Cl2 → Al2Cl6 (s) + 3CO (g)
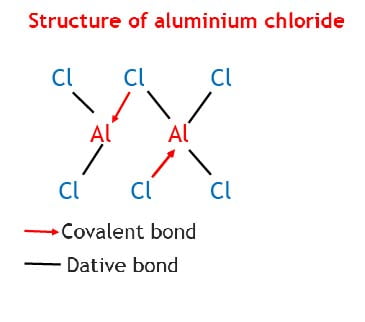
Trial 3
(a) The relative molecular mass of aluminium chloride in a vapour phase is 267.
(i) Write the molecular formula of aluminium chloride in a vapour phase.
(ii) Write a structural formula to show the bonding in aluminium chloride vapour.
(iii) Note the types of bonds involved in the structure you have drawn in (ii) above.
Oxide of aluminium
Preparation
By heating aluminium with oxygen or
by heating aluminium hydroxide
2Al(s) + 3O2 → 2Al2O3 (s)
2Al(OH)3(s) → Al2O3(s) + 3H2O(g)
Please download PDF: Group 3

i like this website.
I love this for I guess one who visits this website for research does not remain the same unless otherwise
Thanks a lot for taking our country to another level
GOD BLESS
You’ve got a unique perspective, and I love it! Bags Wallet & Luggage
Thanks for making complex ideas easy to understand. Real Madrid News
Start playing the Raja Luck Game today and experience the thrill.
Make extra benefits by welcoming good friends with your special Diu Win Invite Code.
Reduce in advance investment while taking full advantage of effectiveness with Server Rental in Bengaluru.
Win huge and improve your skills on the interesting bdg win game.
Enjoy smooth and quick gameplay with 55 club for an unmatched gaming experience.
Get all the latest updates and exclusive perks with the bdg win app.
Find out how to develop a structured Technology Roadmap for seamless IT preparing and execution.
Update your culinary abilities with Best Kitchen Tools And Gadgets developed to make cooking easier and more satisfying.
The quality of downloads from Only MP3 is exceptional, making it my preferred choice for music conversion.
Fantastic ideas for wildlife lovers! I delighted in Corbett more with educated business arranged through Escort Service In Nainital during my trip.
Traditional Recipes need to be preserved and shared worldwide.
Chennai Escort services are discreet.
A Escort Service In Dehradun can offer you the best feelings.
Stay updated with the complete KKR IPL 2025 Schedule, consisting of match dates, locations, and components for Kolkata Knight Riders in the upcoming IPL season.
Couldn’t have had a better trip without Varanasi Trip — so grateful!