Group 3 elements- Inorganic chemistry

Members
Elements symbol
Boron B
Aluminium Al
Gallium Ga
Indium In
Thallium Tl
General comment
Boron is a non-metal, aluminium is a metal whereas gallium, Indium and Thallium are weakly metallic.
Uses of boron
- Boron is used in making heat resistant glasses
- Boron is a essential nutrient.
- Sodium tetra borate (borax), Na2B4O7. 10H2O is used to standardize hydrochloric acid solutions.
Na2B4O7 (aq) + 2HCl (aq) + 5H2O (l) → 2NaCl (aq) + 4H3BO3 (s)
Aluminium
Aluminium is a light metal that strong, malleable and ductile.
Its surface is protected by a thin layer of oxides the prevents from being very reactive.
It is used for electric cables, sauce pans, tins, airplanes.
Extraction of Aluminium
Ore: Bauxite (Al2O3.xH2O)
Major impurities are
- Silica or SiO2
- Iron salts
Principles in extraction
Extraction of aluminium involves removal of impurities (purification) and then reduction to metal by electrolysis.
Steps in extraction of Aluminium from bauxite (Al2O3.xH2O)
(i) The ore is heated
-
- to remove water and,
- To convert iron salts to iron III oxide
(ii) Removal of Iron impurities
The powdered ore is heated with concentrated sodium hydroxide to dissolve aluminium oxide and silica such that the insoluble iron oxide is filtered off.
Aluminium oxide form aluminate
Al2O3 (s) + 2NaOH (aq) + 7H2O (l) → 2Na[Al(OH)4(H2O)2] (aq)
Or the ionic form
Al2O3 (s) + OH–(aq) → 2AlO2–(aq) + H2O(l)
Silica also dissolves forming sodium silicate.
SiO2 (s) + 2NaOH (aq) → Na2SiO3(aq) + H2O (l)
(iii) Separation of aluminium hydroxide from silicon impurities
To the filtrate a little aluminium hydroxide is added to precipitate Aluminium hydroxide,(seeding).
NaAlO2(aq) + 2H2O(l) → NaOH(aq) + Al(OH)3(s)
Alternatively carbon dioxide bubbled through the filtrate to precipitate aluminium hydroxide as follows
2NaAl(OH)4(aq) + CO2(g) → 2Al(OH)3(s) + Na2CO3(aq) + H2O(l)
(iv) Recovering pure aluminium oxide
The precipitated aluminium hydroxide is filtered off, washed and ignited to give pure aluminium oxide (alumina).
2Al(OH)3(s) → Al2O3(s) + 3H2O (g)
(v) Extraction of aluminium from aluminium oxide by electrolysis
Aluminum is obtained from aluminium oxide by electrolysis.
Cryolite, Na3AlF6, is added to aluminum oxide
- lower the melting point of alumina from20500C to 9000C
- and improve conductivity of aluminium oxide
At the cathode (carbon) aluminium is liberated
Al3+ (aq) + 3e– → Al (s)
At the anode (carbon) oxygen is liberated
2O2- – 4e → O2 (g)
The anode is eaten up by oxygen
C + O2 (g) → CO2 (g)
Trial 1
(a) During the extraction of aluminium, the ore is first purified.
(i) Write the name and formula of one ore from which aluminium is extracted. (1 marks)
(ii) Name two main impurities in the ore. (1 marks)
(iii) Name the reagent that is used in the purification of the ore. (1 marks)
Trial 2
The purified ore is mixed with caryolite, melted and electrolyzed.
(i) State the purpose of adding caryolite. (1 mark)
(ii) Name the electrodes used. (1 mark)
(iii) Write an equation for the reaction that takes place at the cathode during the electrolysis. (2 marks)
(iv) Write an equation to show how anhydrous aluminium chloride can be obtained from aluminium. (2 marks)
Trial 3
(i) Write the formula of an ore of aluminium.
(ii) During the extraction of aluminium, the ore is first treated with sodium hydroxide, followed by aluminium hydroxide.
(iii) State the purpose of adding sodium hydroxide. (1.mark)
(iv) Write an equation for the reaction between the ore and sodium hydroxide. (1½ marks)
(v) What is the purpose of adding aluminium hydroxide? (1 mark)
(vi) Briefly explain how aluminium can be obtained after the ore has been treated as in (b).(3.marks)
(vii) Carbon dioxide was used instead of aluminum hydroxide in (b).Write an equation for the reaction that took place.
Reactions of aluminium
- (a) with non-metals
- Aluminium combines directly with oxygen, sulphur, nitrogen and halogens at appropriate conditions.
- 4Al (s) + 3O2 (g) → 2Al2O3(s)
- 4Al (s) + 6S (s) → 2Al2S3(s)
- 2Al (s) + N2 (g) → 2AlN (s)
- 2Al (s) + 3F2 (g) → 2AlF3(s)
The oxide and fluoride are ionic, and the rest are predominantly covalent.
(b) Reaction with HCl
Aluminium reacts with moderately concentrated HCl to give a chloride and hydrogen gas.
2Al (s) + 6HCl (aq) → 2AlCl3(s) + 3H2(g)
(c) with sulphuric acid
Aluminum is not readily attacked by dilute sulphuric acid, but with the concentrated acid it gives the sulphate and sulphur dioxide and water.
2Al (s) + 4H2SO4(aq) → Al2(SO4)3(s)+ 2H2O(l) + SO2(g) + 2H2(g)
(d) Reaction with nitric acid
Aluminium does not react with nitric acid probably due to formation of an impenetrable oxide layer on the surface.
(e) Reaction with sodium hydroxide
Aluminium reacts with sodium hydroxide liberating hydrogen gas.
2Al(s) + 2–OH(aq) + 6H2O(l) → 2Al(OH)4–(aq) + 3H2(g)
Halides of aluminium
(a) Aluminium fluoride (AlF3)
It can be made by reacting metallic aluminium with fluorine. It is the only ionic aluminium halide and it is sparingly soluble in water.
(b) Aluminium chloride, Al2Cl6
Preparation:
- By passing hydrogen chloride or chlorine over heated metal under anhydrous conditions.
2Al(s) + 3Cl2 (g) → Al2Cl6 (s)
2Al(s) + 6HCl (g) → Al2Cl6 (s) + 3H2 (g)
Structure of aluminium chloride
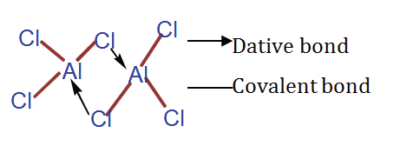
Trial 3
(a) The relative molecular mass of aluminium chloride in a vapour phase is 267.
(i) Write the molecular formula of aluminium chloride in a vapour phase.
(ii) Write a structural formula to show the bonding in aluminium chloride vapour.
(iii) Note the types of bonds involved in the structure you have drawn in (ii) above.
Oxide of aluminium
- Preparation
- By heating aluminium with oxygen or
- by heating aluminium hydroxide
- 2Al(s) + 3O2 → 2Al2O3 (s)
- 2Al(OH)3(s) → Al2O3(s) + 3H2O(g)
Hydrolysis of aluminium salts
Al3+ hydrolyze in solution to produce acidic solution that reacts with carbonate ions to liberate carbon dioxide
Al3+(aq) + 3H2O(l) →Al(OH)3(s) + 3H+(aq)
Consequently
- Aluminium salts solution blue litmus paper red
- Aluminium salts solution liberates carbon dioxide from carbonates and hydrogen carbonates with effervescence.
2Al3+(aq) + 3H2O(l) + 3CO32-(aq) → 2Al(OH)3(s) + 3CO2(g)
Al3+(aq) + 3HCO3–(aq) → Al(OH)3(s) + 3CO2(g)

Very interesting
I’m always impressed by your writing. Garden & Outdoor
You have a gift for making things stick. Sports News