Group 4 element (carbon, silicon, germanium, tin, and lead) (A-level inorganic chemistry)

Group 4 Elements, carbon, silicon, germanium, tin and lead
Members
Elements symbol
Carbon C
Silicon Si
Germanium Ge
Tin Sn
Lead Pb
Physical properties
- Carbon and silicon are nonmetals.
- Germanium is a metalloid
- Tin and lead are metal
Electron configuration
The general electron configuration of group 4 elements is ns2np2
Oxidation states
The common oxidation state of group IV elements is +2 and +4
Stability of oxidation states
Oxidation state +2 become more stable down the group
Oxidation state +4 becomes less stable down the group.
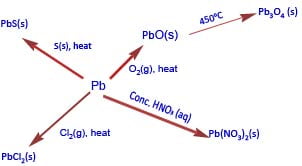
For instance, carbon burns in oxygen to form carbon (IV) oxide (CO2) whereas lead form Lead (II) oxide (PbO).
Secondly, CCl4 is stable whereas PbCl4 easily decomposes on heating giving lead (II) chloride and chlorine
PbCl4 (g) (heat) →PbCl2 (s) + Cl2 (g)
Reasons for variation of stability of oxidation states
Down the group IV; the +2 oxidation state becomes more stable due to the inert pair effect i.e. the two s-electron do not participate in the bonding due to the increase in the energy difference between ns and np electrons.
Properties of the compound with +2 and +4 oxidation states
Compounds in oxidation state +4 are predominantly covalent because formation M4+ requires alot energy whereas compound in oxidation state +2 are ionic
This explains the fact that PbCl4 is soluble in organic solvents like ethanol whereas PbCl2 does not.
Note that
- covalent compounds are soluble in organic solvents whereas ionic compounds do not.
- Covalent compounds have low melting and boiling points whereas ionic compounds have high melting and boiling point.
- Covalent compounds do not conduct electricity whereas ionic compounds do. On this basis compounds in the oxidation state, +4 show properties of covalent compounds because they are predominately covalent.
Chemical properties
Reaction of group (iv) elements with water
Carbon does not react with cold water but reacts with steam to form water gas
C(s) + H2O(g) → CO(g) + H2(g)
Silicon, germanium, and Tin do not react with water up to 1000C because they are protected by thin layer of oxide. But they react with steam at very high temperatures to form dioxides
Ge(s) + 2H2O(g) → GeO2(s) +2H2(g)
Lead reacts with soft water (water that contains oxygen) to form lead II hydroxide.
Pb(s) + 2H2O(g) + O2(g) → Pb(OH)2(s)
Reaction of group (IV) elements with acids
Dilute nonoxidizing acid like HCl.
C, Si, and Ge do not react
Sn liberates hydrogen
Sn(s) + 2H+(aq) Sn2+(aq) + H2(g)
Pb lead does not react with dilute hydrochloric acid and sulphuric acid due to the formation of insoluble salts.
Nitric acid
With concentrated nitric acid.
C, Si, Ge and Sn react to form dioxide
C(s) + 4HNO3(aq)→ CO2(g) +4NO2(g) + 2H2O(l)
Pb reacts to form lead II nitrate and nitrogen dioxide.
Pb(s) + 4HNO3(aq) → Pb(NO3)2(aq) + 2NO2(aq)+ 2H2O(l)
With 50% nitric acid.
Pb reacts to form lead II nitrate and nitrogen monoxide.
3Pb(s) + 8HNO3(aq) → 3Pb(NO3)2(aq) + 2NO(aq)+ 4H2O(l)
With sulphuric acid
Dilute sulphuric acid
C, Si, and Ge do not react.
Sn and Pb reacts dilute concentrated sulphuric acid liberating hydrogen.
Pb (s) + H2SO4(aq) → PbSO4(s) + H2(g)
With hot Concentrated sulphuric acid
C, Si and Ge react to form dioxides
C(s) + 2H2SO4(aq) → CO2(g) + 2SO2(g) +2H2O(l)
Sn and Pb reacts hot concentrated sulphuric acid liberating sulphur dioxide .
Pb(s) + 2H2SO4(aq) → PbSO4(s) + SO2(g) +2H2O(l)
Sn(s) + 2H2SO4(aq) → SnSO4(s) + SO2(g) +2H2O(l)
With acetic or ethanoic acid
C, Si Ge and Sn do not react.
Pb reacts ethanoic acid in the presence of air giving lead II ethanoate and water
Pb (s) + 4CH3COOH(aq) + O2(g) → Pb(CH3COO-)2(aq) +2H2O(l)
Reaction of silicon with hydrofluooric acid or aqueous hydrogen fluoride
Silicon is dissolved by hydrofluoric acid, HF, to form fluorosilicic acid, a reaction apparently driven by the stability of the Si(IV) fluoride complex [SiF6]2-.
Si(s) + 6HF(aq) → 2H2(g) +H2SiF6 (aq) (fluorosilicic acid)
Reaction group (IV) elements with sodium hydroxide
C does not react.
Si Ge and Sn react liberating hydrogen and silicate (IV), germates (IV) and stannates (IV) respectively
Si (s) + 2NaOH(aq) + H2O(l) → Na2SiO3(aq) + 2H2(l)
Lead reacts to form plumbates (II).
Pb(s) + 2NaOH(aq) → Na2PbO2(aq) + 2H2(g)
Unique characteristics of carbon
- Forms gaseous oxides, the rest form solid oxides
Note that
(a) Carbon dioxide is gas because its molecules are bonded by weak van der Waal forces.
(b) Silicon dioxide is a solid of high melting point because each silicon atom is bonded to four oxygen atom by strong covalent bonds.
2. Catenation: carbon forms chains, rings, and branches with single, double and triple bonds while others do not
3. Forms compounds mainly with oxidation state (IV); other elements form compounds with oxidation states +2 and +4.
Carbon tetrachloride and carbon tetrahydride do not hydrolyze in water. Chlorides of other elements hydrolyze in water.
5. Carbon form CF4 and not CF6-2
Reason for the uniqueness of carbon
- Has a very small atomic radius
- Has high electronegativity
- Lacks easily accessible d-orbital in its valence shell.
Allotropes of carbon
Allotropy is the existence of two or more different physical forms in the same state of a chemical element.
An allotrope is any of the different physical forms in the same state into which a chemical element can exist.
Carbon exists in two main allotropes, i.e. graphite and diamond.
Graphite
In graphite, each carbon atom is covalently bonded to 3 carbon atoms to form a layer of hexagons. Each layer is bonded to another by weak van der Waal forces.
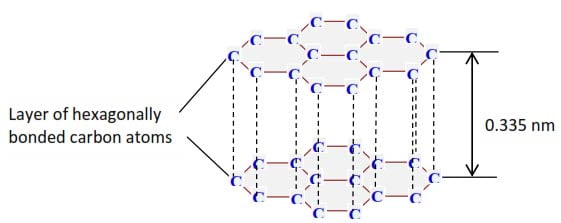
Properties of graphite as a result of its structure
- It has open structure and low density.
- It is slippery and used as a lubricant.
- Has unbonded π-electron that is free to move about making graphite a good conductor of electricity and heat
Diamond
Structure of diamond
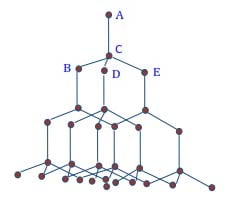
Each carbon atom is bonded tetrahedrally to four carbon atoms to form a 3D compact structure by strong covalent bonds. As a result, the diamond has a high density, melting, and boiling point. It is the hardest substance known.
Diamond is used as an ornament, and to drill and cut other substances.
Differences between diamond and graphite
- The density of graphite (2.3 gcm-3) that of the diamond (3.5 g cm-3)
- Diamond is very hard while graphite is soft
- Graphite is slippery while diamond is not
- Graphite conducts electricity while diamond does not.
Experiment to show that graphite and diamond are allotropes of carbon
When equal masses of graphite and diamond are burned in air, they give an equal volume of carbon dioxide.
Compounds of carbon
1. Hydrides:
they include alkanes, alkenes, alkyne and aromatic compounds studied in organic chemistry
2. Halides of carbon
Carbon forms all the four tetrahalides: CF4, CCl4, CBr4 and CI4
Comments
(a) The melting points of halides increase with molecular. All tetrahalides are volatile liquids
(b) The stability of the halides decreases from CF4 > CCl4 > CBr4 > CI4 due to a decrease in the bond strengths as the electronegativity of the halogen decrease.
(c) CCl4 is a good solvent, being very stable and heavier than air; it is used in fire extinguisher to protect the burning substance from oxygen.
(d) CCl4 is not hydrolyzed by water due to a lack of accessible d-orbital to accommodate the lone pair of an electron from water and weaken the C-X bond.
3. Oxides f carbon
Carbon monoxide (CO)
Preparation
By dehydration of methanoic acid with concentrated sulphuric acid.
HCO2H(l) + Conc. H2SO4(l) → CO(g) + H2O(l)
Uses of carbon monoxide
It reduces most metal oxides to metals.
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
NiO (s) + CO (g) → Ni (s) + CO2 (g)
This property is employed industrially in the extraction of iron and nickel.
It is used to purify nickel: it forms a volatile carbonyl that decomposes to give a pure metal

It is used preparation of carbonyl chloride that is used in the production of polyurethane form of plastics and pesticides.
CO (g) + Cl2 (g) → COCl2 (g)
[carbonyl chloride]
It reacts with fused NaOH under pressure to give sodium methanoate. Methanoic acid is produced from this salt by the addition of dilute HCl.
NaOH (l) + CO (g) → HCOONa (s)
then HCOO- (aq) + H+ (aq) → HCOOH (l)
Methanoic acid is used to produce insecticides and for dyeing, tanning, and electroplating.
Carbon dioxide (CO2)
Preparation
By decomposition of calcium carbonate
CaCO3(s) (heat) → CaO(s) + CO2(g)
Limestone quick lime
Uses of carbon dioxide
- Flavors fizzy drinks.
- It is heavy and stable so it is used in fire extinguishers to displace oxygen from burning objects.
Carbonic acid
Preparation
By reacting carbon dioxide and water. There is, however, equilibrium in the solution of carbonic acid, hydrogen ions, hydrogen carbonate, and carbonate ions.
CO2 (g) + H2O (l) ↔ H2CO3 (aq)
H+(aq) + HCO3– (aq) ↔ 2H+ (aq) + CO32- (aq)
Structure of carbonic acid
Has 3 resonance hybrid whose bond lengths are intermediate between a C=O and C-O bonds
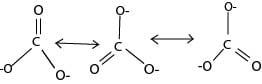
Carbonates and hydrocarbonate
Both react with acids to liberate carbon dioxide
CO32- (aq)+ 2H+ (aq) → H2O (l) + CO2 (g)
HCO3– (aq)+ H+ (aq) → H2O (l) + CO2 (g)
CO32- forms white ppt with Mg2+ whereas HCO3– does not.
Silicon
Existence
Exists as silica, SiO2, or sand used for building or making glass.
Chemical properties of SiO2
Reaction with NaOH
SiO2(s) + 2NaOH(aq) → Na2SiO3(aq) + H2O(l)
SiO2(s) + 2–OH (aq) → SiO32- (aq) + H2O (l).
Chemical properties of SiO2
(a) Reaction with NaOH
SiO2(s) + 2NaOH(aq) → Na2SiO3(aq) + H2O(l)
SiO2(s) + 2–OH (aq) → SiO32- (aq) + H2O (l).
(b) Reaction with HF
SiO2(s) + 4HF(g) → SiF4(aq) + H2O(l)
SiF4(aq) + 2HF(aq) → H2SiF6(aq)
Reaction of silicon with hydrogen fluoride or hydrofluoric acid
Silicon does dissolves in hydrofluoric acid, HF, to form fluorosilicic acid, a reaction apparently driven by the stability of the Si(IV) fluoride complex [SiF6]2-.
Si(s) + 6HF(aq) → 2H2(g) +H2SiF6 (aq) (fluorosilicic acid)
Germanium, tin, and lead
Points to note
a. They form compounds with oxidation states +2 and + 4. The stability of compounds with oxidation states +2 increases down the group due to ‘inert pair effect’ while those of oxidation states +4 decreases down the group.
b. Compounds with oxidation state +2 are reducing. For instance, tin II ions reduce iron III ions to iron II ions.
Sn2+(aq) + Fe3+(aq) → Sn4+(aq) + Fe2+(aq)
c. Compounds with oxidation state +4 are oxidizing. For instance, lead IV oxide ions oxidize HCl ions to chlorine
PbO2 (s)+ 4HCl (aq) → PbCl2(s) + 2H2O (l) + Cl2 (g)
d. Compounds with oxidation state +4 are predominantly ionic whereas those in oxidation state +4 are predominantly covalent. This explains the fact that PbCl2 is soluble in water but insoluble in ethanol whereas PbCl4 is soluble in ethanol
Chemical properties
The principal reactions of germanium and tin are similar, both elements exhibiting oxidation state of 4
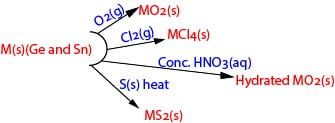
The principal reactions of lead are summarized below
Lead IV halide
Lead forms ionic Pb4+(F–)4, and covalent liquid PbCl4.
The tetra-bromide and tetra-iodide do not exist, presumably because bromine and iodine are not sufficiently strong oxidizing agents to convert Pb2+ to Pb4+.
Lead IV chloride
Preparation
By reacting lead IV oxide with cold concentrated hydrochloric acid.
PbO2 (s) + 4 HCl (aq) (conc. and cold) → PbCl4(aq) +2H2O(l)
Effect of Heat on Lead (IV) chloride
Decomposes on heating liberating chlorine
PbCl4 (g) (heat) → PbCl2 (s) + Cl2 (g)
Hydrolysis of lead (IV) chloride
Lead tetrachloride is readily hydrolyzed by water (white fumes of HCl and brown precipitate of PbO2 are observed)
PbCl4 (s) + 2H2O (l) → PbO2 (s) + 4HCl (aq)
Reaction of lead (IV) chloride with sodium hydroxide
Lead(IV) chloride react with sodium hydroxide to produce lead(IV) oxide, sodium chloride and water. Sodium hydroxide – diluted solution.
PbCl4 + 4NaOH → PbO2 + 4NaCl + 2H2O
The compound PbO2 reacts with the sodium hydroxide to produce the hexahydroxoplumbate (IV) ion [Pb(OH)6]2−, soluble with the water.
PbO2 + 2NaOH + 2 H2O → Na2[Pb(OH)6]
Lead II halide
Preparation
By reacting soluble salt like lead nitrate with soluble halide
Pb2+(aq) + 2X–(aq) → PbX2(s) ( X = F, Cl, Br, I).
E.g. Pb2+(aq) + 2Cl–(aq) → PbCl2(s)
Properties
Except for the iodide, which is yellow, they are white solids. Lead (II) chloride forms chloro-complexes, e.g., [PbCl4]2-, with Cl– ions and because of this, it is soluble in concentrated hydrochloric acid.
Reaction of Lead II chloride with sodium hydroxide
Lead II chloride reacts with sodium hydroxide solution to form lead (II) hydroxide which dissolves in excess sodium hydroxide to form sodium plumbate (II)
PbCl2 (s) + 2OH–(aq) → Pb(OH)2(s) + 2Cl–(aq)
Then
Pb(OH)2(s) + 2OH–(aq) → Pb(OH)42-(aq)
Lead IV oxide
Preparation
Lead (IV) oxide, PbO2, is a brown solid obtained by oxidation of a soluble lead (II) salt with hot sodium chlorate (I).
Pb2+(aq) + H2O(l) + ClO–(aq) → PbO2(s) + Cl–(aq) + 2H+(aq)
(ii) By action of nitric acid on trilead tetra oxide, Pb3O4.
Pb3O4 (s) + 4HNO3 (aq) → 2Pb(NO3)2(aq) + PbO2(s) + 2H2O(l)
Chemical properties of lead (IV) oxide
Decomposes on heating
2PbO2 (s) + heat → 2PbO (s) + O2 (g)
Reacts with cold concentrated hydrochloric acid to form lead (IV) chloride and water
PbO2(s) + 4HCl (aq) → PbCl4(s) + 2H2O(l)
Oxidize hot concentrated hydrochloric acid to chlorine
PbO2(s) + 4HCl(aq) → PbCl2 (s) + 2H2O(l) + Cl2(g)
Reaction of lead (IV) oxide with sodium hydroxide
PbO2 reacts with the sodium hydroxide to produce the hexahydroxoplumbate (IV) ion [Pb(OH)6]2−, soluble with the water.
PbO2 + 2NaOH + 2 H2O → Na2[Pb(OH)6]
Qualitative analysis of Pb2+
- Forms a white precipitate with sodium hydroxide soluble in excess
Pb2+(aq) + 2OH–(aq) → Pb(OH)2 (s) + 2OH–(aq) → Pb(OH)42- (aq)
- Forms white precipitate with ammonia solution insoluble in excess
Pb2+(aq) + 2OH–(aq) → Pb(OH)2 (s)
- Forms yellow precipitate with potassium iodide
Pb2+(aq) + 2I–(aq) → PbI2 (s)
- Forms white precipitate hydrochloric acid-soluble on warming
Pb2+(aq) + 2CI–(aq) → PbCI2 (s)
- Forms white precipitate with sulphate ions
Pb2+(aq) + SO42-(aq) → PbSO4 (s)
Download PDF
Group 4 element (carbon, silicon, germaniun, tin, and lead)

Thanks for the great work in compiling these notes
Thanks a lot the concerned team
Very good work.Kindly include me on your list.Am also a chemistry teacher.l need to be part of the team.
I grateful appreciate
Best regards.
EMITU DAVID
Thanks very much for the great work you are doing, you really helped me in various areas of BCM combination and I really appreciate sir,my colleagues in the same area please subscribe,and download the PDF and video for more help, other wise may God grant you his blessings,!
thanx
Thax very much for compiling those notes they help alot in the chem
Thanks so much team,I really love the way you have summarized it
I’m eagerly awaiting your next post! Tech News
Understand the required scores with the MBBS Cutoff Of Private Medical Colleges in Jharkhand.
Discover qualifying criteria for government institutions at MBBS Cutoff Of Government Medical Colleges in Andhra Pradesh.
Thanks for supplying a clear description of Types Of Backlinks and their influence on search rankings.
Boost your preliminary deposit and enjoy extra funds by entering the Diu Win Invite Code.
Get access to top-tier hardware with flexible Server Rental in Noida that fits your spending plan.
Get the best online video gaming experience with interactive functions on bdg win.
Get instant access to the very best video gaming platform by downloading bdg win app.
Learn how to establish a structured Technology Roadmap for smooth IT planning and execution.
This travel plan is ideal! I ‘d recommend including time for social friendship through Escort Service In Nainital to enhance the experience.
Absolutely nothing can change the Taste Of Home that this location provides.
Discovering the best Chennai Escort can be a game-changer.
Consider utilizing a protected and trustworthy Dehradun Escort Service.