
Heat/enthalpy of reaction (O-level chemistry)

Calculations involving heat in reactions
The reaction involves energy change; exothermic reactions such as burning firewoods produce heat while endothermic reactions such as the dissolution of ammonium chloride in water absorb heat.
The amount of heat absorbed or released in a reaction depend on
- Amounts of the reactants
- Temperature and
- The pressure at which a reaction is carried out.
When molar quantities are involved at 298K and 1 atmosphere, the resultant heat changes are referred to as standard heat/enthalpy changes given a symbol ∆H (delta H).
Enthalpy changes (∆H) (for exothermic reactions carry a negative sign because heat is lost from the system while enthalpy changes (∆H) for endothermic reaction carry a positive sign because heat is gained by the system.
Heat is measured in joules ( J)
Each heat change is identified by names; the common ones are:
- The heat of combustion or enthalpy of combustion of a substance is the heat change when 1 mole of a substance is burnt completely in oxygen.
Experiment to determine enthalpy of combustion A given mass (M1g) of a substance of molecular mass, Mr, is burnt completely in excess oxygen. Heat liberated raises the temperature of (Mw g) of water through a temperature change of θ0


Experimental depends on whether the fuel is a liquid or a solid.
An experimental method for finding enthalpy of combustion a liquid fuel
The figure below shows a simple method for obtaining approximate value for the enthalpy of combustion of a fuel.
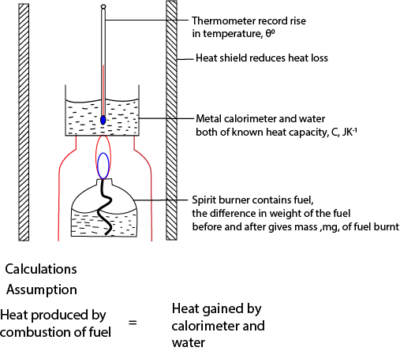
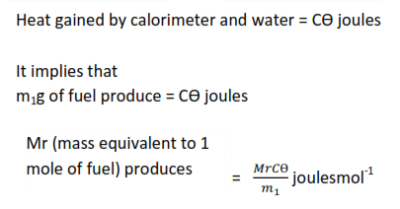
Example 1
When 23g of ethanol completely burnt, 13600KJ of heat was produced.
Calculate the molar heat of combustion of ethanol (C = 12, H = 1, O = 16)
solution
Formula mass of ethanol (CH3CH2OH) = 12 x 2 + 6 + 16 = 46
23g of ethanol liberate 13600KJ
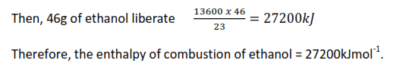
2. Enthalpy of neutralization
This refers to enthalpy change for the formation 1 mole of water from hydrogen and hydroxide ions
H+(g) + –OH(aq) → H2O(l)
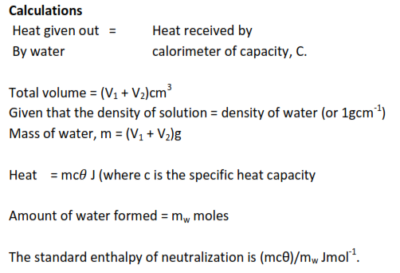
Measurement of standard enthalpy of neutralization
The heat released when a known amount of water is formed is found by measuring the temperature produced in a calorimeter and its contents.

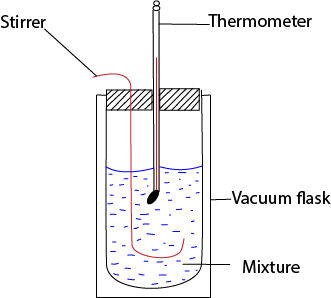
NB. A vacuum flask is used to minimize heat losses
Know volume of standard acid (V1) and alkali (V2) are added to calorimeter, and temperature change θ0C is noted. The number of moles of water formed, Mw, is calculated
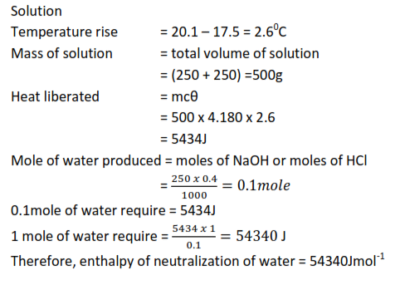
Example 2
250 cm3 of 0.40M NaOH were added to 250cm3 of 0.40M HCl in the calorimeter. The temperature of the two solutions was 17.50C and rose to 20.10C
Calculate the enthalpy of neutralization assuming that the specific heat capacities of a solution are the same as that of water = 4180J kg-1 K-1.
For Revision question and answers please download PDF

Thanks alot your notes really helped me
Nice and understandau
Nicely and understandable
You always make things easy to understand. Electronics
I’m excited to see where you’ll go next. Indian Cricket
Learn about the latest MBBS Cutoff Of Government Medical Colleges in Delhi for top institutions.
Learn about hassle-free enrollment at MBBS Direct Admission in Delhi.
Enjoy exclusive benefits by applying the Raja Luck Invite Code.