
Iron and its compound (A-level inorganic chemistry)

Iron and its compound
Electron configuration of Iron
[Ar]3d64s2
Properties of iron as a transition element
- Forms coloured compounds; for instance Fe2+ is green
- Has variable oxidation states; +2, +3,
- Forms complexes, e.g. Fe(CN)63-.
- Has catalytic properties, e.g., iron catalyze formation of ammonia from nitrogen and hydrogen.
Extraction of iron
Iron, which is the second most abundant metal occurring in the earth’s crust, is extracted from its ores which are;
haematite, Fe2O3
magnetite, Fe3O4,
siderite, FeCO3.
Extraction of iron from hematite
Main Impurities
Silica, SiO2
Procedure
Iron ore, limestone and coke in the correct proportions are reacted with
Preheated air in a blast furnace.
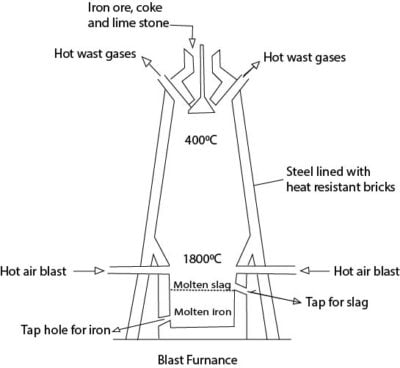
Reactions leading to reduction of iron oxide are
C (s) + O2 (g) →CO2 (g)
CO2 (s) + C (s) →2CO (g)
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
Reactions leading to removal of impurities
CaCO3 (s) → CaO (s) + CO2 (g)
CaO (s) + SiO2 (s) → CaSiO3 (l)
CaO (s) + Al2O3 (s) → CaSiO3 (l) slag
Molten iron and slag percolate to the bottom of the furnace and taped of through different taps.
Trial 1
In the production of iron metal, haematite, Fe2O3, is mixed with coke and limestone and heated in a blast furnace.
(a) What is the purpose of adding
(i) limestone (1 mark)
(i) coke? (1 mark)
(b) Write three different equations for the reaction in which haematite are converted into iron in the blast furnace. (3marks)
(c) Explain why it is possible to extract iron by the method described above. ( 2 marks)
(d) (i) Name one other method that can be used to extract iron from its ore. ( 1 marks)
(ii) Suggest a reason why the method you have named in d(i) is not used commonly in the production of iron.
Oxidation states of Iron
Iron has oxidation states +2 and +3
Oxidation state Fe3+ ([Ar]3d5) is more stable than F2+ ([Ar]4s13d5) because Fe3+ has stable half-full electron configuration.
Trial 2
This explains why green iron II compounds rapidly turn into brown iron III compounds.
Properties of iron
Pure iron is a silvery colored metal with a melting point of 15350C and boiling point 30000C.
It is a soft, ductile and malleable metal, which is strongly ferromagnetic.
Rusting of iron
Iron in presence water and air form a brown coat called rust or hydrated iron (III) oxide, Fe2O3.xH2O,
Disadvantage of rusting
- Weaken tools
- Makes objects look ugly
Methods of preventing rusting
(a) Keeping objects in dry places.
(b) Painting
(c) Oiling
(d) Greasing
(e) Coating with another metal.
(f) Zinc plating
The reaction of iron with water
Iron does not react with cold water but with steam to form blue-black tri-iron trioxide oxide
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
Reaction of iron with acids
Iron displaces hydrogen readily from dilute hydrochloric acid and sulphuric acids, forming iron (II) salts. Dilute nitric acid forms iron (II) nitrate and oxides of nitrogen.
Fe(s) + 2HCl(aq) → FeCl2 (aq) 2H2(g)
Fe(s) + H2SO4(aq) →FeSO4(aq) + H2(g)
Concentrated H2SO4 gives a mixture of ferrous and ferric sulphates.
Fe(s) + 2H2SO4(aq) → FeSO4(aq) + SO2(g) + 2H2O(l)
2FeSO4 (aq) + 2H2SO4 (aq) → Fe2(SO4)3 (aq) + 2H2O (l) + SO2 (g)
Action of alkalis, halogen and sulphur on iron
Iron does not react with alkalis.
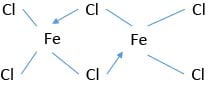
Halogens and sulphur combine with the heated metal forming halides and sulphide respectively.
2Fe(s) + 3Cl2(g) → 2FeCl3 (g)
Fe(s) + S(s) → FeS (s)
Displacement of less electropositive metals
Iron displaces copper from a solution of copper sulphate.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
Hydrolysis of Iron III slats in water
Iron III salts hydrolyze in water to form an acidic solution
Fe3+(aq) + 3H2O(l) → Fe(OH)3(s) +3H+(aq)
Consequently, Iron III solution
- turns blue litmus paper red.
- Liberate carbon dioxide from carbonates with the formation of brown precipitate
- Has pH less than 7
Reaction iron III solution with sodium carbonate solution
2F3+(aq) + 3CO32-(aq) + 3H2O(l) → 2Fe(OH)3(s) + 3CO2(g)
Observation
A brown ppt. formed with effervescence
Trial 3
State what would be observed and write an equation for the reaction that takes place when aqueous iron (III) chloride with sodium carbonate (3½ marks)
Reactions of iron (III) as an oxidizing agent
Iron (III) compounds are strong oxidizing agents, for instance, it oxidizes iodide ions (I–) to iodine and tin (II) compounds to tin (IV) compounds and itself reduced to Fe2+ compounds.
2Fe3+(aq) + 2I–(aq) → 2F2+(aq) + I2(aq)
2Fe3+(aq) + Sn2+(aq) → 2F2+(aq) + Sn2+(aq)
Trial 4
A solution of Iron (III) chloride was added to an aqueous solution of tin (II) chloride acidified with hydrochloric acid and the mixture shaken.
(a) State what was observed. (1 mark)
(b) Write an equation for the reaction that took place.
Qualitative analysis of Fe2+ salt
Common iron II salts include
- Ferrous sulphate: FeSO4.7H2O
- Ammonium ferrous sulphate (NH4)2Fe(SO4)2.6H2O
- Ferrous oxalate. FeC2O4
- Addition of dilute sodium hydroxide or ammonia solution
Observation
A dirty green precipitate insoluble in excess turn brown on standing.
Fe2+(aq) + 2OH–(aq) → Fe(OH)2(s)
- Confirming presence of Iron III ions
Forms a deep blue solution with potassium hexacyanoferrate II.
Forms a red solution with ammonium thiocyanate solution.
Watch this
Sponsored by The Science Foundation College +256 753 80 27 09
Compiled by Dr. Bbosa Science

This is exactly what I was looking for. Industrial & Scientific
I love how informative and concise this is. Barcelona News
Discover admission trends with the MBBS Cutoff Of Government Medical Colleges in Orissa.
Discover cost-effective programs with MBBS Fees Structure in Uttar Pradesh.
Start playing the Raja Luck Game today and experience the thrill.
Stay competitive with additional benefits by utilizing the Raja Games Invite Code.
Thank you for this useful method to Backlink Strategy, it’s really handy!
Drive long-term SEO development with Build backlinks for website.
Take pleasure in smooth deals and unique discounts with the Diu Win Invite Code.
Get access to top-tier hardware with flexible Server Rental in Noida that fits your budget.
Engage in non-stop enjoyable and excitement on bdg win game.
Experience smooth online gaming with bdg win and enjoy awesome obstacles.
Only MP3 delivers excellent audio quality while keeping the file sizes small.
Your photography catches Nainital completely! I have stunning memories too, some produced with buddies from Escort Service In Nainital throughout my stay.
I’m glad to have actually discovered South Bangalore‘s finest source of genuine food.