
Kinetic theory (O-level chemistry)

KINETIC THEORY
States that “mater is made of small particles that are in a continuous random motion”
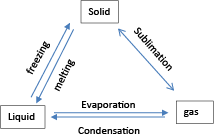
The particles move fastest in gases and slowest in solids. This is because the intermolecular forces in solids are stronger than in liquids than in gases.
When a solid is heated, the particles gain kinetic energy and turns into a liquid by a process called melting or into a gas by the process called sublimation.
Heating a liquid turns it into a gas by the process called evaporation.
Cooling a gas turns into a solid by the process of sublimation or into a liquid by condensation and a liquid turns into a solid by freezing or solidification.
The summary for interconversion of phase of matter
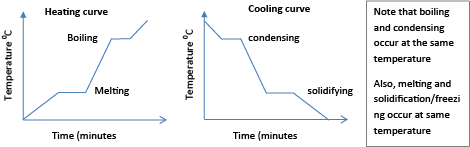
Note that melting/solidification and boiling/condensing occur at constant temperatures. The characteristic heating and cooling curves are shown below;
Evidence of kinetic theory
- Diffusion of gases.
This is the movement of particles from areas of high concentration to a region of low concentration.
2. Brownian motion
This is the random movement of smoke particles in a glass cell as seen through a microscope.
Explanation
Air particles are in random movement; these collide with smoke particles and cause them to move randomly.
When the temperature is increased, the kinetic energy of the air particles increases which increases the speed of smoke particles.
The Brownian motion can be observed by the dust particles through light rays
3. Formation of white fumes of ammonium chloride (NH4Cl) from ammonia (NH3) and hydrogen chloride gas (HCl) from hydrochloric acid (HCl)
When cotton wool soaked in ammonia and another soaked in concentrated hydrochloric acid are placed in the opposite end of a long glass tube, a white ring form in a tube near the side that contains cotton wool soaked in concentrated hydrochloric acid.
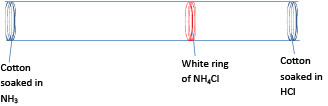
Conclusion
- Ammonia and hydrochloride acid contains particles that move to meet each other and react to form white fumes of ammonium chloride
- Ammonia and hydrochloric acid are volatile i.e. can easily turn into vapor.
- The white ring forms near the cotton wool soaked in HCl because ammonia molecules are lighter and therefore diffuse/move faster. Or ammonia molecules move a bigger distance than hydrogen chloride molecules at the same time.
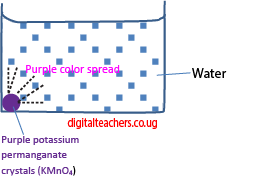
4. The presence and movement of particles in liquids is demonstrated by diffusion of purple permanganate color in water.
For revision question and answers download the link below

Your insights always hit the mark. Tech News
Access premium colleges via MBBS Admission Through Management/Nri Quota in Haryana.
Understand qualifying benchmarks at MBBS Cutoff Of Government Medical Colleges in Haryana.