
Lands Berger’s method of determining boiling point elevation constant of a solvent

Lands Berger’s method of determining boiling point elevation constant of a solvent
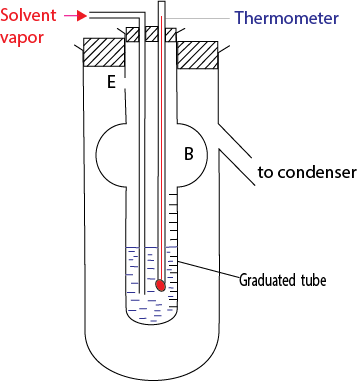
- The solvent of boiling point, T0, is boiled in a separate flask and used to boil the solution.
- Excess vapor escapes through the hole E.
- The boiling point of the solution T10C is obtained from the thermometer.
- The final volume of the liquid in the graduated tube is read off and from the density of the solvent the mass (ms g) of the solvent into which a solute of mass m2 is dissolved is calculated.
Then the boiling point constant Kb is given by
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES Inorganic chemistry questions
TAGS Dr. Bbosa Science


You have a real talent for this subject. Computer & Accessories
You have a gift for explaining things clearly. Top adult movies
The Top MBBS Colleges in Madhya Pradesh are paving the way for the next generation of medical leaders.
Download the Raja Luck App to enjoy seamless gaming on your mobile device.
Get an expert overview of Raja Luck and its importance.