
Molecular structures (shapes molecules) (A-level inorganic chemistry)

Molecular structures (shapes of molecules)
This chapter provides the principles used to predict the shapes of molecules of different compounds.
Understanding molecular shapes is very vital because enzyme actions and their inhibitors and activity of many drugs (especially antibiotics) depend exclusively on their molecular structures.
Diatomic molecules, like H2, Cl2, O2, HCl, HF are linear in shape.
However, for polyatomic molecules, i.e., H2O, CH4, CO2, NH3, etc., the shapes adopted by their molecules depend on: –
1. The total number of electron pairs around the central atom.
2. The number of bonding electrons and lone pairs.
The valence shell electron pair repulsion theory (VSEPR theory)
To predict shapes of polyatomic molecules, this theory puts into consideration the number of bonding and lone pairs of electrons around the central atoms and the repulsion between the electron pairs.
NB: A lone pair of electrons is found on one atom only; it is under the influence of one nucleus whereas the bonding pair is under the influence of two nuclei. Therefore, the lone pairs of electrons are free compared with the bonding pairs and occupy plenty of space. As lone pairs are closer to the central atom, they cause greater repulsion than the shared (bonding) pairs and the repulsion decreases in this order.
Lone pair – lone pair > lone pair-bond pair > bond pair-bond pair.
Predicting shapes of polyatomic molecules
Using the VSEPR theory, we will consider cases in which the central atom is surrounded by a total of two to six electron pairs.
Case 1
Total number of electron pairs around the central atom = 2.
Number of bonding pairs = 2.
Number of lone pairs = 0.
The molecule will be of the form AX2 and is linear in shape.

Case 2
Total number of electron pairs around central atom = 3
Two possibilities:
a) Total number of electron pairs around the central atom = 3.
Number of bonding pairs = 3.
Number of lone pairs = 0.
The molecule will be of the form AX3.
Shape: Triangular or trigonal planar, bond angle 1200.
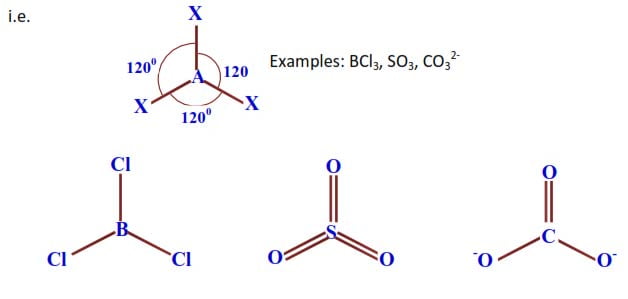
b) Total number of electron pairs around the central atom = 3.
Number of bonding pairs = 2.
Number of lone pairs = 1.
The molecule will be of the form AX2 but with a lone pair
Shape: Angular /V-shaped.

Case 3
Total number of electron pairs around central atom = 4
Three possibilities:
a) All the four pairs are bonding pairs.
No. of lone pairs = 0
Form of the molecule = AX4
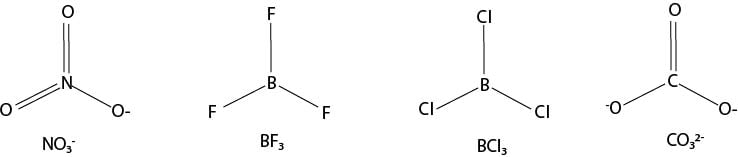
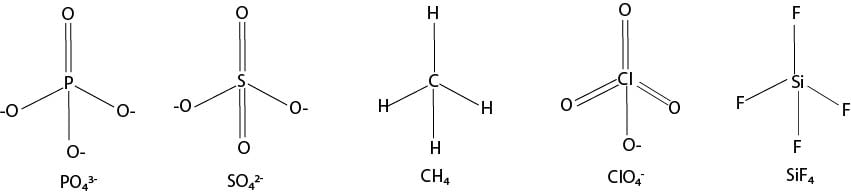
Shape: Tetrahedral, bonding angle = 1090.30′ or 109.50

Examples, CH4, CCl4, SO42-, PO42-, MnO4-, NH4+
b) Total number of electron pairs around the central atom = 4.
Number of bonding pairs = 3.
Number of lone pairs = 1.
The molecule will be of the form AX3 but with a lone pair of electrons.
Shape: Trigonal Pyramidal.

The bond angle XÂX, depends on how close to or far from the central atom, the shared pairs of electrons are. The closer they are to the central atom, the stronger the repulsion between them and hence the larger the bond angles, e.g., NH3 (ÐHNH = 1070), PH3 (ÐHPH = 930 20′), AsH3 (ÐHAsH = 910 50′), and SbH3 (ÐHSbH = 910 50′).
The decrease in the bond angle in passing from NH3 to SbH3 is due to the decrease in electronegativity of the central atom. The electronegativity of the central atoms decreases in the order: N ñ P ñ As ñ Sb.
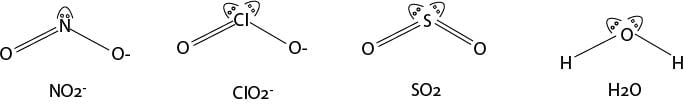
c) Total number of electron pairs around the central atom = 4.
Number of bonding pairs = 4.
Number of lone pairs = 2.
The molecule will be of the form AX2 but with two lone pairs.
Shape: Angular

Case 4
Total number of electron pairs around central atom = 4
(a) Total number of electron pairs = 5
Number of lone pairs = 0
Number of bond pairs = 5
Formula AX5 e.g. PCl5
Shape: Trigonal bipyramidal

Case 5
Total number of electron pairs around central atom = 6
(a) Total number of electron pairs = 6
Number of lone pairs = 0
Number of bond pairs = 6

Formula AX6 e.g. SiF6, [Al(H2O)6]3+
Shape: octahedral
NB: Structures of molecules that do not involve lone pairs on the central atom are highly symmetrical, i.e., linear, triangular, and tetrahedral. This is because such structures involve only one kind of repulsion (bond pair-bond pair) and in such structures, there is a constant bond angle, i.e., linear – 1800, triangular = 1200, and tetrahedral 109.50, triangular bipyramidal, octahedral, etc.
Common shapes in examination
The triangular planar molecule has uniform repulsion among bond pairs; bond angle is 1200.
Triangular pyramidal shape: there is strong repulsion between pair and bond pair
Tetrahedral shape: uniform repulsion among bonding pairs; bond angle 109.50
Angular:
Trial 1
(i) Sketch the shapes of the following molecules. (3marks)
(i) NH3 (ii) BF3 (iii) H2S
(ii)Briefly explain why each molecule adapts the shapes in (a) above. (6marks)
Trial 2
Sketch the following molecules, SO2Cl2, PO43-, ClO4–, MnO4–, ClF3, ClO3–, ClO2–,NO2–,NO3–, SiF4, (CH3)3N, CO32-, NCl3, PCl5
Watch this
Sponsored by the Science Foundation College + 256 753 802709
Compiled by Dr. Bbosa Science

Thanks for the work done I needed bonding and structure video
you tube search: bonding Dr. Bbosa Science
This is spot on. Keep up the good work! Car & Motorbike
Congratulations Dr ,will i also find ict alevel?
You really know your stuff. Sports News
Simplify your enrollment with MBBS Admission Through Management/Nri Quota in Rajasthan.
Discover cost-effective programs with MBBS Fees Structure in Uttar Pradesh.
The Raja Luck App Download gives you access to premium games.