
Nitrogen and it compounds (O-level chemistry)

Nitrogen and its compounds (O-level)
Source: Air
Industrial preparation of nitrogen
By distillation of air from which carbon dioxide and water vapor have been removed. Nitrogen distills of leaving oxygen as the residue. (oxygen has a higher boiling point than nitrogen)
Laboratory preparation
By oxidation of ammonia with copper oxide
Set up
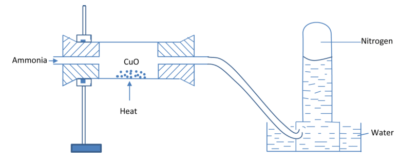

Equation
2NH3(g) + 3CuO (s) → N2 (g) + 3H2O(l) + 3Cu(s)
Physical properties on nitrogen
- It is colorless
- Odorless
- Nonreactive
Chemical properties of nitrogen
Nitrogen does not react easily because the nitrogen atoms are bonded by strong triple covalent bonds
However, under drastic conditions, it reacts to form compounds. For instance, nitrogen reacts with magnesium and lithium to form nitrides. This is because the formation of nitrides liberates a lot of heat that breaks the strong nitrogen-nitrogen triple bond
3Mg(s) + N2(g) → Mg3N2 (s)
6Li(s) + N2 (g) → 2Li3N(s)
The nitrides hydrolyze in water to give ammonia
Mg3N2(s) + 6H2O(l) → 3Mg(OH)2 (s) + NH3 (g)
Ammonia is an alkaline gas that turns litmus solution blue or damp red litmus paper blue.
Note that magnesium burns in air to produce magnesium oxide as well.
Mg(s) + O2(g) → 2MgO(s)
Magnesium oxide is a white solid that is insoluble in water.
Exercise 1
Magnesium burns in air to produce two solids A and B. A is insoluble in water while B reacts with water to form a gas that turns damp red litmus paper blue
- Identify Solids A and B.
- Write equations leading to the formation of A and B.
- Write an equation between B and water.
Uses of nitrogen
- The raw material for synthesis of ammonia and nitric acid
- Coolant
Compounds of nitrogen
Ammonia, NH3
Industrial preparation
By reaction nitrogen and hydrogen
N2(g) + 3H2(g) ↔ 2NH3(g) + heat
Conditions for the production of ammonia.
- Pressure:
Production of ammonia is accompanied with a decrease in the number of moles of a gas and as such high yield of ammonia is favored by high pressure.
2. Temperature
Production of ammonia produces heat; high yields are thus expected at low temperatures.
However, at low temperature, the reaction is slow that a compromise temperature of 4500-5000C is used.
3. Catalyst is iron speed up the reaction
Laboratory preparation of ammonia
By reacting calcium hydroxide (Ca(OH)2) with ammonium chloride (NH4Cl)

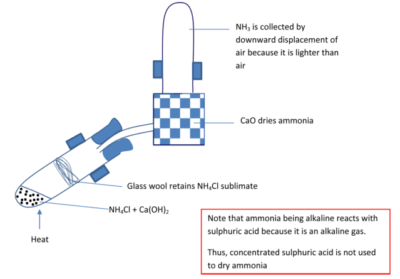
Equation
Ca(OH)2 (s) + 2NH4Cl (s) → CaCl2(aq) + 2H2O (l) + 2NH3 (g)
Properties of ammonia
(i) Has chocking smell
(ii) Turns damp red litmus paper from red to blue.
(iii) It is very soluble in water
This solubility is demonstrated by a Fountain experiment.
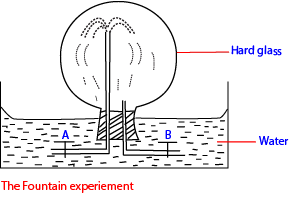
(a) The hard glass flask is filled with ammonia and taps A and B are closed
(b) The flask is inverted in water as shown above.
(c) Tap B is open water dissolves ammonia to create a vacuum inside the flask
(d) Immediately tap A is open, water splashes into the flask like a fountain.
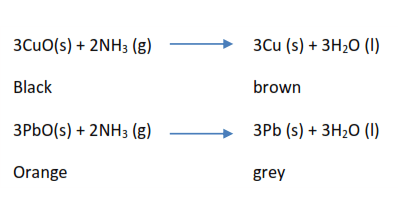
(iv) Ammonia as a reducing agent
It reduces black copper oxide to brown copper and orange lead II oxide to grey lead metal.
(v) Oxidation of ammonia
Ammonia burns with a blue flame in oxygen.

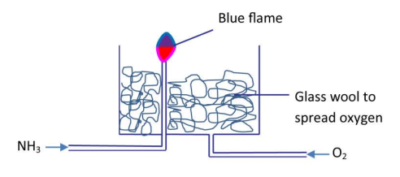
Equation
4NH3 (s) + 3O2 (s) → 2N2(g) + 6H2O (l)
In presence of excess O2 and platinum or copper wire catalyst, ammonia is oxidized to nitrogen monoxide and the brown fumes on nitrogen dioxide.
4NH3 (s) + 5O2 (s) → 4NO(g) + 6H2O (l)
2NO2 (s) + O2 (s) → 2NO2(g)
(vi) The reaction of ammonia solution with common cations
(a) Form a white precipitate with lead (II) and aluminium salts that are insoluble in excess
NH3 (aq) + H2O (l) → NH4+(aq) + OH– (aq)
Then
Pb2+(aq) + 2OH–(aq) → Pb(OH)2(s)
Al3+(aq) + 3OH–(aq) → Al(OH)3(s)
(b) Forms white precipitate with zinc II salts soluble in excess due to formation of a complex ion
Zn2+(aq) + 2OH–(aq) → Zn(OH)2 (s)
Then
Zn(OH)2 (s) + 4NH3 (aq) (excess) → Zn(NH3)42+(aq)
(c) Forms blue precipitate with copper II salts soluble in excess to give a deep blue solution due to formation of a complex ion
Cu2+(aq) + 2OH–(aq) → Cu(OH)2 (s)
Then
Cu(OH)2 (s) + 4NH3 (aq) (Excess) → Cu(NH3)42+(aq)
Uses of ammonia
- Disinfectant
- For the preparation of liquid soap
- Fertilizers
Note that prolonged use of ammonia nitrate fertilizer may make the soil acidic because ammonium nitrate in soil reacts with water forming ammonium hydroxide and nitric acid plants absorb ammonium ions leaving nitric acid in solution.
NH4NO3(s) + H2O(l) → NH4OH (aq) + HNO3 (aq)
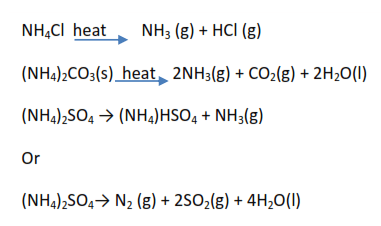
Ammonium salts
Common ammonium salt include ammonium chloride (NH4Cl), ammonium sulphate ((NH4)2SO4) ammonium carbonate ((NH4)2CO3) and ammonium nitrate (NH4NO3)
- Ammonium chloride, ammonium carbonate, and ammonium sulphate decompose to give alkaline gas ammonia (turns damp red litmus paper blue). Ammonium chloride sublimes the rest do not.
2. Ammonium salts react with sodium hydroxide to liberate ammonia
NH4+(aq) + OH–(aq) → NH3(g) + H2O(l)
Ammonia is an alkaline gas that turns damp red litmus paper blue.
Nitric acid
Industrial preparation
Ammonia is oxidized to nitrogen dioxide in the presence of platinum catalyst.
4NH3 (s) + 7O2 (s) → 4NO2(g) + 6H2O (l)
The nitrogen dioxide is dissolved in water in presence of oxygen to produce nitric acid
4NO2 (g) + 2H2O (l) + O2 (g) → 4HNO3 (aq)
Laboratory preparation
By heating potassium nitrate with concentrated sulphuric acid
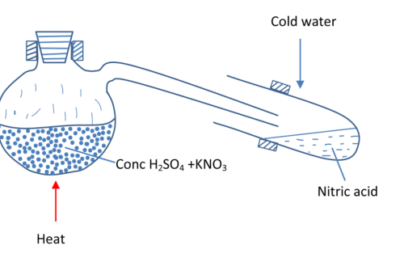

Equation
H2SO4 (aq) + 2KNO3 (s) → HNO3(aq) + KHSO4(aq)
Properties of nitric acid
- Turns blue litmus red
- Liberate carbon dioxide from carbonates and hydrogen carbonates
HCO3– + H+ (aq) → CO2(g) + H2O(l)
CO32- + 2H+ (aq) → CO2(g) + H2O(l)
3. It reacts with metals to produce hydrogen
Mg (s) + 2H+(aq) → Mg2+(aq) + H2(g)
4. It oxidizes nonmetals
S(s) + 6HNO3(aq) → H2SO4 (aq) + 6NO2(aq) + 2H2O (l)
C(s) + 4HNO3(aq) → CO2 (s) + 6NO2(aq) + 2H2O (l)
5. It decomposes on heating to form nitrogen dioxide and oxygen
4HNO3(l) (Heat) → 4NO2 (g) + O2 (g) + 2H2O(l)
Nitrogen dioxide is soluble in water where oxygen is insoluble in water. Therefore, when fuming nitric acid is heated, the gas collected over water is oxygen.
6. It oxidizes metals
It oxidizes copper and lead to copper II nitrate and lead II nitrate respectively.
3Cu(s) + 8HNO3(aq) (dil) → 3Cu(NO3)2(aq) + 2NO(g) + 4H2O (l)
3Pb(s) + 8HNO3(aq) (dil) → 3Pb(NO3)2(aq) + 2NO(g) + 4H2O (l)
Cu(s) + 4HNO3(aq)(conc.) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O (l)
Pb(s) + 4HNO3(aq) (conc) → Pb(NO3)2(aq) + 2NO2(g) + 2H2O (l)
Nitrates
These are salt of nitric acid
Decomposition of nitrates
- Nitrates of group 1 elements decompose on heating to give nitrites and oxygen
2NaNO3 (s) → 2NaNO2 (s) + O2 (g)
2. Nitrates of other elements decompose to liberate oxide, nitrogen dioxide, and oxygen.
2Cu(NO3)2 (s) → 2CuO(s) + 2NO2(g) + O2 (g)
2Pb(NO3)2 (s) → 2PbO(s) + 2NO2(g) + O2 (g)
3. Silver nitrate decomposes on heating to liberate silver metal, nitrogen dioxide, and oxygen
2Ag(NO3)2 (s) → 2Ag (s) + 2NO2(g) + O2 (g)
4. Ammonium nitrate decomposes on heating into dinitrogen oxide and water
NH4NO3 → N2O(g) + 2H2O(l)
Testing for nitrates
- Nitrates are identified from the brown fumes when nitrates are heated strongly.
- Brown ring test
To 1 cm3 of a nitrate solution, add freshly prepared iron II sulphate, followed sulphuric acid slowly over the wall of a slanted test tube.
Observation
A brown ring forms at the junction of the two solutions.
Uses of nitric acid
For fertilizers, dyes, explosives.
For sample questions and answers download PDF below

Brief notes to follow
Good work
Thanks for posting this work, it has really helped me. May you continue with that.
Very good and precise Chemistry notes for students’ understanding. Thanks.
It is good.
Very good chemistry notes.Thank you for nice job
its good for learning.
God bless you for your endavour
This is spot on. Keep up the good work! Home Improvement
You have a real knack for this. Indian Football
The MBBS Admission Through Management/Nri Quota in Karnataka ensures premium education opportunities.
Secure direct access to premier institutions with MBBS Direct Admission in Rajasthan.
Start playing the Raja Luck Game today and experience the thrill.
Get a deeper understanding of Raja Luck and its opportunities.
Upgrade your information storage and processing power with Server Rental in Delhi.
I’m preparing my trip next month and will certainly consider Escort Service In Nainital based on these recommendations. Thanks!
The benefits on bdg win are worth the time invested playing.
Get the latest news and updates in **Hindi News**, covering sports, innovation, and entertainment.
The fan edits and trending clips on https://www.youtube.com/@kpopbuzzindo/shorts are always top-notch!