
Period table and bonding (O-level chemistry)

The Periodic Table (PT)
The periodic table is a tabular arrangement such that the columns contain elements with electronically similar atoms.
The rows of the periodic table are called periods while the vertical columns are called groups.
There are 7 periods and 8 groups.
Some special groups
Group I or alkali metals
This consists of the elements, H, Li, Na, K, Rb, Cs, and Fr; except hydrogen, these elements are highly metallic in character
Group II or alkaline earth metals.
This consists of the elements, Be, Mg, Ca, Sr, Ba, and Ra. All these elements are metals but their metallic character is less than that of group 1.
Group VII or halogens
This consists of elements, F, Cl, Br, I, and At. All these elements are nonmetals.
Group VIII or noble elements, inert elements or zero elements
Consists of the elements, He, Ne, Ar, Kr, Xe, and Rn. and are all gaseous elements at room temperature.
Summary
The extreme left end of the periodic table consists of metals with the exception of hydrogen; the extreme right end consists of non-metals. The middle part of the periodic table consists of semi-metals or metalloids.
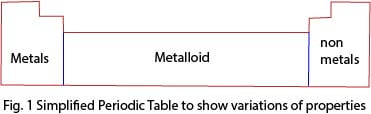
Trends in physical properties of elements in the periodic table
- The nonmetallic character increases from left to right of the periodic table.
- Atomic size decrease from left to the right due to the increase in nuclear attraction.
- Ionization energy is the energy required to remove an electron from a gaseous atom to form positively charged gaseous ion. Ionization energy increases across the period and for this reason, metal atoms easily lose electron whereas nonmetal atoms do not. Generally, metals react by loss of electrons forming positive ions whereas nonmetals react by gaining electron forming a negative ion.
Bonding
A bond is the force of attraction that binds the atoms within a molecule.
There are four types of chemical bonding namely: –
1. Ionic (electrovalent) bonding.
2. Covalent bonding.
(a) Normal covalent bonding.
(b) Dative (coordinate) bonding.
3. Metallic bonding.
4. Van der Waals forces
Elements react to attain stable (doublet or octet) electronic configurations of the noble gases.
Ionic bonding
It is usually formed between metal and non-metals
This involves the transfer of one or more electrons from one atom (metal) to another (nonmetal), e.g.
Na → Na+ + e–
(2:8:1) (2:8)
Cl + e– → Cl–
(2:8:7) (2:8:8)
Or

By losing one electron, sodium atom achieves a stable electronic structure (similar to that of neon) while the addition of an electron to a chlorine atom makes it achieve a stable electronic configuration (similar to that of argon).
It is the electrostatic attraction resulting from the opposite charges that constitute the ionic or electrovalent bond between Na+ and Cl– ions in sodium chloride.
Characteristic properties of ionic compounds
- They consist of ions and not molecules.
- They are electrolytes, i.e., when dissolved in water or when fused, conduct an electric current and decompose into constituent ions.
- They are solids of high melting points.
- They are insoluble in organic solvents but many of them are soluble in water.
Covalent bonding
This is divided into two types, namely, the normal covalent bond and the dative or a coordinate bond.
- The normal covalent bonding
This involves the sharing of one or more pairs of electrons to attain stable electron configurations similar to those of noble gases. Considering a chlorine atom, which has seven electrons in its outermost shell, if one electron is provided by each atom and shared equally, then each chlorine atom can acquire a complete octet configuration as it forms a chlorine molecule. The shared pair of electrons constitutes a covalent bond.
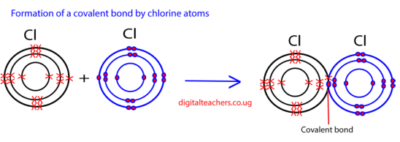
Characteristics of covalent compounds
a) They consist of discrete molecules.
b) They are non-electrolytes.
c) They have low boiling and melting points.
d) They are insoluble in water and other polar solvents but soluble in organic or nonpolar solvents.
- Dative or coordinate bonding
The dative bond is like a covalent bond once formed except that both electrons in the shared pair are provided by one atom. The atom providing the two electrons is called the donor and the atom which accepts the two electrons is the acceptor. The donor atom must have an unshared pair of electrons available and such a pair of electrons is called a lone pair. An example is the reaction of ammonia with H+ or AlCl3. By reacting with ammonia, H+ attains a doublet structure of helium whereas Al3+ attains an octet configuration of argon.
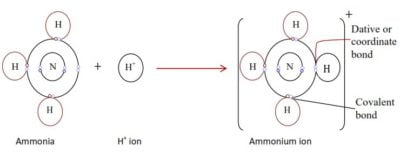
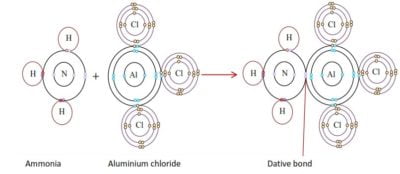
Metallic bond
Arrangement of ions and delocalized electrons in metals. Pure metals are made up of atoms, all of the same kind; each atom losses its valence electrons and becomes a positive ion. The lost electrons are free to move and they are called delocalized electrons.
A metal, therefore, consists of positively charged atoms surrounded by its valence electrons, which are free to move about within the solid. The free or delocalized electrons attract the positively charged ions thus binding them together equally and stronger. At the same time, the positively charged ions attract the delocalized electrons preventing them from dispersing, resulting in a metallic bond. These electrostatic attractions bind the entire crystal as a single unit. This is illustrated below:
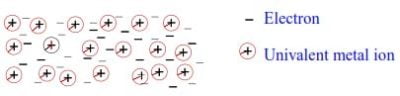
Properties of metals
- They conduct electricity due to the presence of delocalized electrons. When the voltage is applied across the metal, the delocalized electrons are able to move freely through the structured conducting an electric current.
- Metals generally have high densities because the ions are closely packed in the lattice.
- They usually have high melting and boiling points due to the strong attraction between the positive metal ions and the delocalized electrons. Most non-metals have low melting points and boiling points.
- They are malleable and ductile.
Malleable: means that metals can be harmed into different shapes
Ductile: means that the metals can be pulled out into thin wires. Unlike those in diamond, the bonds are not rigid but are still strong. If a force is applied to a metal, rows of ions can slide over one another. They reposition themselves and the strong bonds reform.
Molecular or van der Waals bond
These are intermolecular forces that occur between nonpolar molecules as a result of momentarily induced dipoles in neighbouring molecules. These are a weaker type of bonding whose magnitude increases with the size or mass of the molecules. The melting points of hydrides of group 5 elements from phosphorus and the boiling points of halogens from chlorine to iodine all increase down the group due to the increase in molecular mass.
Van der Waals forces are weak forces the reason why substances bound by these forces like iodine have low melting and boiling points. In fact, iodine sublimes at room temperature.
Giant atomic structure
Also called giant molecular structures. Examples are diamond and graphite (both comprising of carbon atoms only), silicon dioxide (silicon IV oxide)
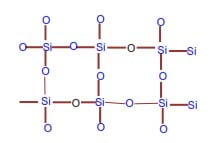
Giant atomic structure of silicon dioxide
Giant ionic structure e.g. sodium chloride

Giant ionic structures consist of many oppositely charged ions held together by the electrostatic force of attraction e.g. Sodium chloride. In sodium chloride each sodium ion has six equidistant chloride ions around it and arranged octahedrally as shown above.
Substances with giant ionic structures have high melting points because a large amount of energy is needed to break the structure due to strong electrostatic forces between the ions.
For revision questions and answers, download PDF

Thank you so much members of digital teachers, especially Dr.bbosa
We are so happy for your services
Keep on updating us and our learners at large
Very good blog post.Thanks Again. Fantastic.
Thanks for being a beacon of knowledge. Health & Personal Care