
Periodic Table (A-level Inorganic chemistry)

The Periodic Table (PT)
The periodic table is a tabular arrangement such that the columns contain elements with electronically similar atoms.
The rows of the periodic table are called periods while the vertical columns are called groups. There are 7 periods and 8 groups.
The layout of the periodic table demonstrates recurring (periodic) chemical and physical properties.
Elements are listed in order of increasing atomic number (i.e. the number of protons in the nucleus). Rows are arranged so that elements with similar properties fall into the same columns (group)
Some special groups
Group I (1A)
This consists of the elements, H, Li, Na, K, Rb, Cs, and Fr; except hydrogen, these elements are highly metallic in character and are known as alkali metals.
Group II (2A)
This consists of the elements, Be, Mg, Ca, Sr, Ba, and Ra. All these elements are metals but their metallic character is less than that of group 1. These elements are known as alkaline earth metals.
Group VII ( 7B)
This consists of elements, F, Cl, Br, I, and At. All these elements are nonmetals and are known as halogens.
Group VIII (0)
Consists of the elements, He, Ne, Ar, Kr, Xe, and Rn. These elements are non-reactive and are known as the noble elements, inert elements, or zero elements and are all gaseous elements at room temperature.
Summary
The extreme left end of the periodic table consists of metals with the exception of hydrogen; the extreme right end consists of non-metals. The middle part of the periodic table consists of semi-metals or metalloids (fig. 1).
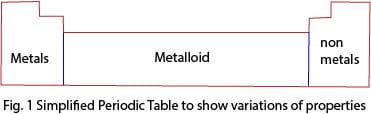
Therefore, the metallic characteristics of the elements decrease across the period, i.e., (left-right).
The periodic table commonly used by Uganda National Examinations Board is given in table 2.1 below.

According to the quantum mechanical theories of electron configuration within atoms, each row (period) in the table corresponds to the filling of quantum shell of electrons. There are progressively longer periods further down the table. And elements are grouped into s, p, d– and f-blocks to reflect their electronic configurations.
Trends in physical properties of elements in the periodic table
The physical properties to be considered include:
1. Electronegativity/Electropositivity.
2. Metallic character.
3. Atomic sizes/atomic radii.
4. Ionic size/ionic radius.
5. Ionization energy.
6. Electron affinity.
7. Melting points/boiling points.
Electronegativity
In a covalent bond between unlike atoms, the shared pair of electrons is not shared equally, because the atom with a greater attraction to electrons pulls the shared electrons towards itself. The measure of the power of an atom to attract bonding electrons is termed electronegativity. Or electronegativity of an element in a molecule is its relative tendency (or power or ability) to attract the shared/bonding pair of electrons towards it. Electronegativity of elements increases across a period as shown in table 2.
Table 2 Variation of electronegativity across the second period of the periodic table
| Elements of 2nd period: | Li | Be | B | C | N | O | F |
| Electronegativity (arbitrary units): | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
NB: Fluorine, the most electronegative element, is given an arbitrary value of 4 and the electronegativity of the atoms of other elements is related to it.
Reason
Electronegativity increases across a particular period from alkali metal to halogen (table 2.2). Because there is a progressive increase in effective nuclear charge that exerts a contracting effect on the outer electron shells, (i.e., in the same period the additional electron resides in the same shell.)
The electronegativity decreases down a particular group (table 3). This is because the atom becomes progressively larger and the screening effect by electrons on inner shells decreases the attraction between the positive nucleus and the peripheral electrons.
Table 3 Variation of electronegativity down group 1 of the periodic table
| Elements of group 1A: | Li | Na | K | Rb | Cs |
| Electronegativity (arbitrary units): | 1.0 | 0.9 | 0.8 | 0.8 | 0.7 |
Trial 1
(b) Explain what is meant by the term electronegativity.
State the factors that determine the value of electronegativity of an element.
(c) Explain how the following factors affect the value of electronegativity of an element.
(i) atomic radius,
(ii) nuclear charge,
(iii) the screening effect of the inner electrons.
Electropositivity
This is the measure of the ease of an atom of an element to lose valence electrons. Electropositivity decreases across each period, due to an increase in the effective nuclear attraction on the valence electrons. Electropositivity increases down each group due to the decrease in effective nuclear attraction on the valence electrons as the atomic sizes and screening effect increase. Thus, group IA elements are the most electropositive elements.
Trial 2
Explain the variations in the electropositivity of the following elements
- C, Ge, and Sn.
- Mg, Al, P and Cl. (8marks)
Metallic character
Since the metallic character depends on how easily an element loses its valence electrons, the metallic character decreases across the period, due to the increase in the effective nuclear charge on the valence electrons. Metallic character increases down a group. This is due to the decrease in the effective nuclear charge on the valence electrons.
Atomic radii
Atomic radius is the distance from the center of the nucleus to the furthest electron under the influence of a nuclear charge of an atom.
The radius of an atom is determined chiefly by two factors.
1. The attraction of positively charged nuclei for the valence electrons: Strong attractions result in smaller sizes/radii of the atoms as valence electrons are pulled closer to the nucleus.
2. The screening of the outer electrons from the nucleus by those from the inner shells: The greater the screening effect, the bigger the size/radius of the atom. Therefore, atomic radii decrease across a period (table 2.4) due to increase in effective nuclear charge and increase down the group (table 2.5) due to the increase in the screening effect which decreases the effective nuclear charge on the valence shell, e.g.,
Table 4 Variation of atomic radii across the second period of the periodic table
| Elements of period 2A: | Li | Be | B | C | N | O | F |
| Atomic radii (nm)x10 | 1.23 | 0.89 | 0.80 | 0.77 | 0.70 | 0.66 | 0.64 |
Table 5 Variation of atomic radii down group 2 of the periodic table
| Elements of group 2A: | Be | Mg | Ca | Sr | Ba |
| Atomic radii (nm) x 10: | 0.89 | 1.36 | 1.74 | 1.91 | 1.98 |
Thus, screening has its greatest effect on the alkali metal atoms that contain only one electron in their outermost shells. Because of this, these atoms have the largest atomic radii in their respective periods.
Variation of atomic radii in transition elements
The atomic radii of elements of a particular series decrease gradually up to the midway element (from Sc to Mn) and then these values remain almost constant up to the elements of group 1B (Fe-Cu). The last element of each series i.e. Zn shows an increase in atomic radius.
Reason
For elements from Sc to Mn, the atomic radii decrease because of the gradual increase in nuclear charge with an increase in atomic number. From Fe to Cu, the atomic radii remain almost constant because electrons added to the d-orbital screen the 4s electron(s), and the attraction between the nucleus and 4s electrons decreases. The additional nuclear charge is counterbalanced by the screening effect.
Towards the end of the series, there is an increase in electron-electron repulsion between the electrons added to 3d and 4s orbitals. This increase in repulsion becomes greater than the attraction between the nucleus and the 4s electrons, thus, because of the greater magnitude of electron-electron repulsion, the atomic size of Zn is greater than that of Cu.
Trial 3
(a) Explain what is meant by the atomic radius. (4marks)
(b) Describe and discuss the change in radii in the following series;
(i) Na, Mg, Al, Si, P, S and Cl (7 marks)
(ii) F, Cl, Br and I (4 marks)
(iii) Ti, V, Cr, Fe and Co (4 marks)
Trial 4
(a) What is meant by the term ‘atomic radius’? (4marks)
(b) Explain how atomic radii vary;
(i) Along a short period of the periodic table
(ii) Down the group in the periodic table (8marks)
Trial 5
The atomic numbers and atomic radii of some transition metals are given in the table 2.6 below
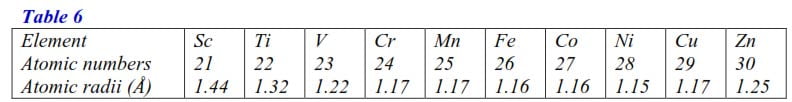
(i) Plot a graph of atomic radius versus atomic number (4 marks)
(ii) Explain the shape of your graph. (7 marks)
Ionic radii
The variations of the ionic radii across the different periods and down the groups are similar to those of the atomic radii as demonstrated by the examples in tables 7 and 8 respectively.
Table 7 Variation of ionic radii across the second period of the periodic table.
| Ions | Li+ | Be2+ | B3+ | O2- | F– |
| Ionic radius(nm) x10 | 0.78 | 0.31 | 0.20 | 1.40 | 1.36 |
Table 8 Variation of ionic radii down group 1 of the periodic table.
| Ions | Li+ | Na+ | K+ | Rb+ | Cs+ |
| Radius(nm) x10 | 0.78 | 0.095 | 0.133 | 0.148 | 0.165 |
NB: (a) Atomic radii are very much smaller than anionic radii but very much larger than cationic radii (table 9).
Table 9 Comparison of atomic and ionic radii of cation and anion.
| Atoms | Ions |
| Na 0.157 nm | Na+ 0.095 nm (cation) |
| Cl 0.099 nm | Cl– 0.181 nm (anion) |
- The radii of iso-electronic positive ions, (i.e., cations with the same number of electrons, e.g. both Li+ and Be2+ have the same number of electrons), decrease with increasing positive charge. There is only a small change in ionic radii for iso-electronic negative ions with a change in negative charge e.g. Cl– and S2- in table 2.10.
Table 10 Comparison of ionic radii of iso-electronic ions.
| Cations | Anions |
| Li+ 0.060 nm | S2- 0.184 nm |
| Be2+ 0.031 nm | Cl– 0.181 nm |
Trial 6
(a) Explain how the sizes of ionic radii of group II ions affect the properties of their compounds compared to those formed by group I metals. (8 marks)
(b) Explain the following observations.
(i) When moving from sodium (0.189 nm) to chlorine (0.099 nm) along period 3 of the periodic table, there is steady decrease in the size of covalent radii.
(ii) Although K+ and Cl– ions both have 18 electrons, the ionic radius of K+ is 0.132 nm while that of Cl– is 0.181 nm.
Variation of ionic radii of transition metals
(a) Since the transition metals show many oxidation states, the ionic radii of the ions of different oxidation states are different.
(b) Generally, the ionic radii of different cations of the same element in different oxidation states decrease with an increase in oxidation state, e.g.
Cr2+>Cr3+>Cr4+>Cr5+>Cr6+
This is due to increase in effective nuclear charge.
(c) Ionic radii of cations of different elements in the same oxidation state generally decrease with the increase in nuclear charge or an atomic number e.g.
Fe2+>Co2+>Ni2+>Cu2+
Ionization energy (I.E)
This is the energy required to remove one electron in the ground state completely from a gaseous atom or ion. The ionisation energy thus, may be classified as first, second, third ionisation energies, etc. for example,
A(g) → A+(g) + e ΔH = 1st I.E
A+(g) → A2+(g) + e ΔH = 2nd I.E
A2+(g) → A3+(g) + e ΔH = 3rd I.E
Variation of the first ionization energy across a period
The first ionization energies of the first 20 elements of the PT plotted against their atomic numbers are shown in the graph below (fig. 2):
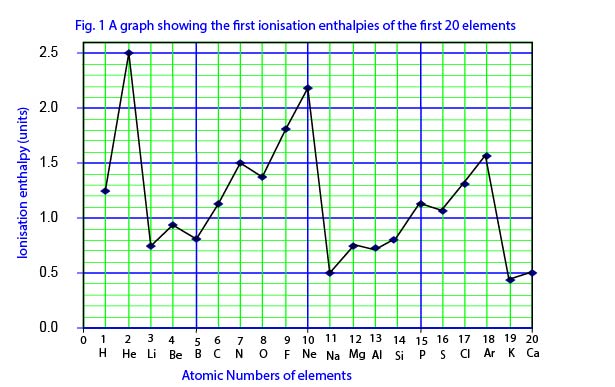
From lithium to neon there is a pronounced increase in ionization energies with slight breaks occurring at positions occupied by boron and oxygen. An exactly similar trend occurs in the portion of the graph from sodium to argon.
Reasons for the two trends
- The increase in the first ionization energies across the period is due to an increase in the effective nuclear charge on the valence electrons as the number of electrons on the outer shell increases.
- Half filled (ns1, np3 nd5) or completely filled (ns2np6nd10) orbitals are comparatively more stable, and hence more energy is needed to remove an electron from them. This explains why the first ionization energies of beryllium (full orbital, 1s2 2s2) and nitrogen (half full orbital, 1s2 2s2 2p3) are higher than those of boron and oxygen respectively.
- The sharp decrease in ionization energies from helium to lithium is explained by the fact that the outer electron in lithium is farther from the nucleus than in helium. Also, the removal of an electron from helium leads to a less stable half-full orbital while the removal of an electron from lithium leads to the formation of a stable full orbital 1s2.
Variation of the first ionization energies down a group
For elements in the same group, the ionization energies decrease down the group, due to an increase in the screening effect or decrease in the effective nuclear charge because electrons are added to a new shell. For example, the first ionization energies of lithium, sodium, and potassium are 520, 494, and 418 kJ mol-1 respectively.
Trial 2.7
(a) Study the graph (fig.2) of the first ionization energies against atomic numbers for the elements in the third short period of the periodic table given below and answer the questions that follow.
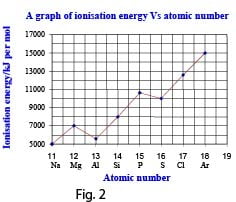
(i) What is meant by first ionization energy? Illustrate your answer using the sodium atom. (3 marks)
(ii) Explain the general trend in the first ionization energies of the elements shown in the graph.(3 marks)
(iii) The first ionization energies of Al and S are lower than expected. Explain (5 marks)
(b) Describe the trend in the first ionization energies down a group of the periodic table and explain your answer. (5 marks)
(c) Show how the first ionization energies of the elements in a group are related to their reactivities.
Illustrate your answer using the reactions of group I elements with water. (4 marks)
Trial 8
(a) Define the term ionization energy.
(b) The first ionization energies of elements in period III of the periodic table and atomic numbers are given in table 2.11 below
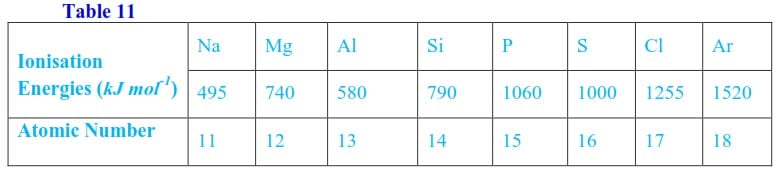
Plot a graph of ionization energy against the atomic number and explain the shape of the graph. (9 marks)
(c) The melting points of magnesium, silicon and sulphur are 6500C, 14230C and 1200C respectively. Explain the difference in melting points of the elements. (6 marks)
(d) Name the type of bonding that exists in the hydrides of Na, P and S. (1½ marks)
(e) Write equations to show the reactions, if any, of the hydrides in (i) with water. (2½ marks)
Trial 9
The graph in figure 2 shows the variation of the first ionization energies of the elements in the first row of the periodic table
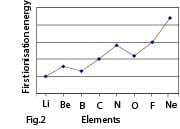
Explain the following observations:
(i) There is a general increase in first ionization energy from lithium to neon. (3marks)
(ii) The first ionization energy of beryllium is higher than that of boron.
(iii) The first ionization energy of oxygen is lower than that of nitrogen.
Variation of successive ionization energies of an atom
Very convincing evidence for the arrangement of electrons into definite shells is available from tabulated values of successive ionization energies of atoms; those for the potassium atom are shown in fig. 3. The ionization energies cover a very wide range of values and for the convenience of graphical plotting, their logarithmic values are used.
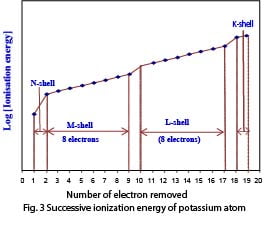
The clear-cut increases in ionization energy are observed when the 2nd, 10th and 18th electrons are involved, which indicates quite clearly that the nucleus of the potassium atom is surrounded by electrons grouped into a number of shells.
Successive ionization of electrons becomes difficult, since every time an electron is removed; the atom carries one more unit of a positive charge.
However, the large increases in ionization energy for removal of the second 2nd, 10th and 18th electrons suggest that the 2nd electron is closer to the nucleus than the 1st electron; similarly the 10th and 18th electrons are nearer to the nucleus than the 9th and 17th electrons respectively. Thus, electrons of a potassium atom can be grouped into shells as follows (table 2.12):
Table 2.12 Arrangement of electrons in the potassium atom
| SHELL | K | L | M | N |
| NUCLEUS 19p, 20n | 2 | 8 | 8 | 1 |
The electronic configuration of potassium is thus, written as 1s2 2s2 2p6 3s2 3p6 4s1.
The properties of elements are closely linked to their ionization energies. The magnitude of the ionization energy is the measure of the metallic character of the elements. The first ionization energies for metals are nearly all below 800 kJ mol-1 whereas those of non-metal are nearly all above 2000 kJ mol-1.
Trial 10
Explain the following observations
- Although ionization energies generally increase across a period in the periodic table, the first ionization energy of boron is less than that of beryllium. (5marks)
- Both valence electrons of magnesium occupy the same energy level yet the second ionization energy of magnesium is greater than its ionization energy. (3marks)
- Calcium forms compounds containing Ca2+ ions, but none containing Ca+ even though its first ionization energy is lower than the second ionization energy.
Trial 11
(a) The 1st, 2nd, 3rd and 4th ionization energies of aluminum are 577, 1816, 2745, 11573 kJ mol-1 respectively.
Explain the trend in the ionization energies. (3 marks)
(b) Aluminium forms stable compounds in which aluminium has an oxidation state of +3 but not of +2 or +1. Explain this observation.
Trial 12
(a) Write the electronic configuration of strontium. (1 mark)
(b)What is the principal oxidation state of strontium? (1mark)
(c) State whether the first ionization energy of strontium is greater or less than that of barium. Explain your answers.
Trial 13
The diagram below shows successive ionisation energies for an element X, showing removal of all its electrons.
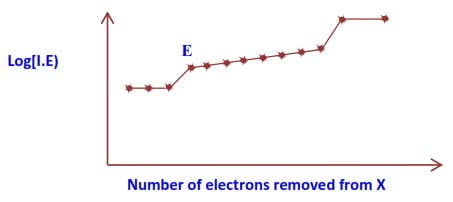
(a) Giving reasons, state:
(i) The group of element X.
(ii) The period of element X.
(iii) Identify element X. (4 marks)
(b) Explain the sudden increase in the energy required to remove electron E. (2 marks)
(c) Explain how the size of X will change as electrons are removed. (1 marks)
Trial 14
The first eight ionisation energies in kJ mol-1 of an element B are shown below (table 13).
Table 13
| 1st I.E | 2nd I.E | 3rd I.E | 4th I.E | 5th I.E | 6th I.E | 7th I.E | 8th I.E |
| 786 | 1580 | 3230 | 4360 | 16000 | 20000 | 23600 | 29100 |
(i) Explain what is meant by the term first ionization energy? (2 marks)
(ii) State the factors that determine the value of the first ionization energy and explain how they affect this value. (3 marks)
(iii) To which group in the periodic table does element B belong. Explain your answer. (1 mark)
Trial 15
Table 214 below shows the ionization energies (in kJ mole-1) of five elements A, B, C, D and E.
Table 14
| Elements | 1st I.E | 2nd I.E | 3rd I.E | 4th I.E |
| A B C D E |
500 740 630 900 580 |
4600 1500 1600 1800 1800 |
6900 7700 3000 14800 2700 |
9500 10500 4800 21000 11600 |
(a) Which one of these elements is most likely to form an ion with a charge of +1? Give a reason for your answer.
(b) State:
(i) Two elements which belong to the same group in the periodic table.
(ii) The group to which the elements you have stated in (b)(i) belong.
(c) (i) Write the formula of the chloride of element E.
(ii) Write an equation for the reaction between the chloride of element E and water.
Electron affinity
This is the energy involved in adding an electron to a gaseous atom. It is the energy change for the process.
A (g) + e– → A– (g) ΔH = electron affinity.
Electron affinities of elements increase across a period due to increase in the effective nuclear charge (or electronegativity) and decrease down a group due to the decrease in the effective nuclear charge on the valence shell. Halogens have the highest values of electron affinities. However, the electron affinity of fluorine is lower than that of chlorine. This is due to the fact that the size of fluorine atoms is small, thus the incoming electron is repelled by the nine electrons that are close to each other in the valence shell. The successive electron affinities become more and more endothermic because the incoming electron is repelled by the electrons already in the atom; therefore, additional energy is required to add this electron.
Trial 16
(a) Explain the difference between electronegativity and electron affinity.
(b) The first electron affinities of group VII elements are given in table below.
| Element | F | Cl | Br | I |
| 1st electron affinity / kJ mol-1 | -328 | -349 | -325 | -295 |
(i) Explain what is meant by the term electron affinity.
(ii) Explain the trend in the electron affinities.
(iii) The first and second electron affinity values for the oxygen atom are -121 kJ mol-1 and +744 kJ mol-1 respectively.
Explain why the second electron affinity is endothermic.
Melting points of elements
The melting point of a substance or element is the measure of energy required to break down the regular arrangement of atoms, ions or molecules in the substance (solid state). Therefore, the melting point indicates the strength of the forces knitting atoms, ions or molecules together in the crystal. The strength of those forces in elements varies according to the type of crystals formed and due to this; the melting points of elements in a period do not change uniformly, (e.g table 15 below).
Table 15 The melting points of elements of the third period of the periodic table
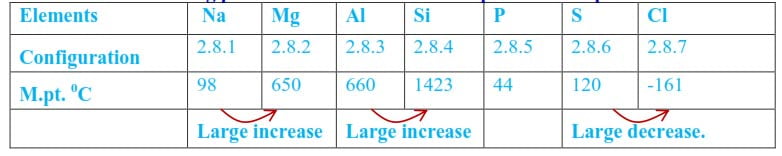
From sodium to aluminium, the melting point increases due to increase in the strength of metallic bonds.The strength of metallic bonds increases as the number of electrons contributed to the formation of the metallic bond increases. Sodium contains one loosely bound electron in the valence shell, and this electron is readily contributed to the formation of the metallic bond. Magnesium contains two electrons in the valence shell and both electrons are contributed to the formation of metallic bond. The metallic bond in magnesium is thus stronger than that in sodium, which explains the large difference in the melting points.
However, the increase in melting point from Mg to Al is not as sharp as expected, probably because aluminium atoms use only two electrons of three valence electrons in the formation of the metallic bond.
Silicon has the highest melting point because it uses its four valence electrons to form an infinite three-dimensional assembly of atoms linked by a strong single covalent bond; thus, high temperatures are required to break these strong bonds.
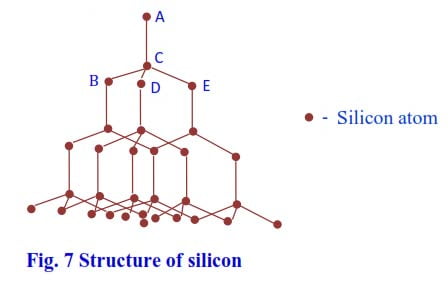
In the structure, each silicon atom is tetrahedrally bonded to four silicon atoms with a crystal unit of five silicon atoms (ABCDE) in fig. 7 (the structure similar to that of diamond). Such a structure is described as a three-dimensional giant structure. As the melting of silicon involves breaking of the strong covalent bonds, the melting point of silicon is thus very high.
The melting points of P, S, Cl are dependent on the sizes/masses of the molecules formed. The melting points of phosphorus and sulphur are relatively higher than expected because they exist as P4 and S8, rather than simple individual atoms; thus, the intermolecular forces are stronger. The boiling point of chlorine is very low because it exists as discrete diatomic molecules, Cl2 held together by weak van der Waals forces.
Trial 17
The atomic numbers and the melting points of some elements in period 3 of the periodic table (table 2.15) are shown below.
Table 15
| Element | Na | Mg | Al | Si | P |
| Atomic number | 11 | 12 | 13 | 14 | 15 |
| Melting point/0C | 98 | 650 | 660 | 1410 | 44 |
(a) (i) Plot a graph of melting points against atomic numbers. (3 marks)
(ii) Explain the shape of the graph in (i). (6 marks)
(b) Describe and explain how the oxides of magnesium, aluminium and silicon react with
(i) sodium hydroxide.
(ii) hydrochloric acid. (9marks)
(c) State the type of bonding in the oxides of sodium and phosphorus. (2marks)
Trial 18
(a) The melting points of magnesium, silicon and sulphur are 6500C, 14230C and 1200C respectively. Explain the difference in melting points of the elements. (6 marks)
(b) Name the type of bonding that exists in the hydrides of Na, P and S. (1½ marks)
(c) Write equations to show the reactions, if any, of the hydrides in (i) with water. (2½ marks)
Download PDF below
Watch this

Thanks for the great work of providing chemistry papers and their guides, the helped to greatly improve in my chemistry….
What’s Happening i am new to this, I stumbled upon this I’ve discovered
It absolutely helptul and it has aieed me out loads. I aam hoping to contribute & help other users like its aided me.
Great job. https://Www.Waste-Ndc.pro/community/profile/tressa79906983/
What’s Happeningg i am new to this, I stumbled upon this I’ve discovered It
abssolutely helpful and it has aded me out loads.
I am hoping to contribute & help other users like its aided me.
Great job. https://Www.Waste-Ndc.pro/community/profile/tressa79906983/
I couldn’t agree more. Well said! Home Improvement
You have a real knack for this. Transfer Latest
Plan your medical education journey with the MBBS Fees Structure in Sikkim.
Understand the entry standards at MBBS Cutoff Of Private Medical Colleges in Karnataka.
Enhance your gaming skills by playing on Raja Luck.
Discover why Raja Luck is gaining attention.
Enjoy secure and swift transactions for deposits and withdrawals within Goa Games, ensuring a hassle-free gaming experience.
Enhance your earnings by utilizing the Referral Code provided by TS EARN.
Open exclusive rewards by utilizing the Diu Win Invite Code when signing up.
Find end-to-end IT options with versatile Server Rental in Mumbai.
Take part in non-stop fun and enjoyment on bdg win game.
Hello i am kavin, its my first occasion to commenting
anyplace, when i read this paragraph i thought i could also make comment due to this sensible paragraph. https://caramellaapp.com/milanmu1/_zJdh2nHV/artemis-prostate-fusion-biopsy
Hello i am kavin, its my first occasion to commenting anyplace, when i read this paragraph i thought i could also make comment due to this sensible paragraph. https://caramellaapp.com/milanmu1/_zJdh2nHV/artemis-prostate-fusion-biopsy
If you would like to improve your experience simply keep visiting this website and be updated
with the latest gossip posted here. https://wakelet.com/wake/y9-7dogSI1hFhn7oxMIzN
If you would like to improve your experience simply keep visiting this website and be updated with the latest gossip posted
here. https://wakelet.com/wake/y9-7dogSI1hFhn7oxMIzN