
Photocells

The Photocells
- Photocells change radiation into electric current.
- In their construction, the anode is made thin so that it does not obstruct the incident radiation
- It’s placed in vacuum because the metals are reactive
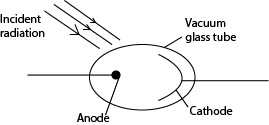
- When radiations fall on the cathode, electrons are emitted and these electrons are collected by the anode.
- If the anode is positive respect to the cathode, current flows in the circuit
Types of Photocells
(a) Photo emissive cells
(b) Photovoltaic cells
(c) Photoconductive cells.
Photo emissive Cell

- When radiation of frequency f greater than f0 (threshold frequency) of the photo emissive cathode is incident on the cathode, electrons are emitted, they move to the anode and current flows in the external circuit.
- The size of the current increases with the intensity of the incident radiation.
- If the light beam is interrupted, the current stops flowing.
- When the device is connected to a suitable relay circuit, it can be used to open doors, act as a burglar alarm or as switching device.
Photovoltaic Cell
It generates an e.m.f dependent on the intensity of the incident radiation. Such cells are used in solar panels, solar calculators and for powering electronic watches.
Photoconductive Cell
It consists of a plate of a material called photoconductor, whose resistance decreases when it is illuminated by light or infrared radiation, mounted in an evacuated glass bulb. An applied voltage causes current to flow which increases with the intensity of the radiation due to release of electrons in the photoconductor.
Experiment to show the variation of current (I) with p.d (V)

- A monochromatic light i.e. constant frequency is used.
- The photocurrent (I) is measured for increasing values of V at constant light intensity.
- For negative values of V, the polarity of the battery is reversed.
- The experiment is repeated by increasing the intensity of the radiations; by moving the light source closer to the photocell
- A plot of graphs of photocurrent (I) against the p.d V is shown below.

N.B: The photocurrent is not zero even when the p.d is zero. This is because electrons are emitted with varying velocities (K.E), some of which are sufficient to overcome the repulsive electric field and reach the anode.
Example 3

A photocell is connected in the circuit as shown in figure (I) above. The cathode is illuminated with monochromatic light of wavelength 390nm and the current I in the circuit recorded for different p.d V applied between the anode and the cathode. The graph fig (II) shows the results obtained.
(a) Find the maximum K.E of the photoelectrons
From the graph Vs = -1V
K.Emax = eV = 1.0 x 1.6 x 10-19 = 1.6 x 10-19
(b) What is the work function of the cathode in eV?

(c) If the experiment is repeated using monochromatic light of wavelength 310nm, where would the new graph cut the V-axis?

Hence the graph would cut the v-axis at – 1.83V
Applications of Photocells
- Photoelectric Emission)
- A photocell can make doors open automatically in buildings when a light beam is interrupted by somebody/obstacle.
- Intruder alarm systems. The intruder intercepts the infrared beam falling on a photocell, hence cutting off of current. This interruption therefore sets the alarm on.
- Photovoltaic cells are used in solar panels, calculators and for powering electronic watches.
- Used as automatic devices for switching on light at night when it tries to darken or when the frequency of the light reduces.
- Automatic counting machines in industries.
- Production of sound from a film
Differences between x-ray production and photoelectric effect:
| Photoelectric effect | X-rays |
| Electromagnetic radiation falls on metal surface and electrons are emitted | Fast moving electrons hit the metal target and x-rays (electromagnetic radiation) is produced |
| Little heat is generated | A lot of heat is generated |
Example 4
A 100mW beam of light of wave length 4.0 x 10-7m falls on a caesium surface of a photocell.
(i) How many photons strike the caesium surface per second?

(ii) If 80% of the photons emit photoelectrons. Find the resulting photocurrent.
Number of electrons emitted = 2 x 1017s-1 x 80%
= 1.6 x 1017
Current = ne
= 1.6 x 1017 x 1.6 x 10-19
= 2.56 x 10-2A
(iii) Calculate the kinetic energy of each photoelectron if the work function of caesium is 2.15eV .

Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much

I’m always learning something new from you. Indian Cricket