
Preparation of gases (O-level chemistry)

Gases
LABORATORY PREPARATION OF GASES
Four main stages involved when preparing a gas in the laboratory.
- Production stage: here the reaction occurs in the reaction vessel to liberate a gas.
- Purification stage; involves the removal of any impurities from the gas.
- Drying stage: it is often necessary to prepare dry gases.
Common drying agents include:
(i) Concentrated sulphuric acid (H2SO4).

N.B: concentrated sulphuric acid is not used to dry ammonia because ammonia (NH3) is basic can react with the acid.
(ii) Anhydrous calcium chloride (CaCl2).

N.B: anhydrous calcium chloride is suitable for drying most gases except ammonia which react with it.
(iii) Quicklime (calcium oxide)

N.B: Calcium oxide (CaO) is usually used to dry ammonia (NH3)
Dried gases must not be collected over water.
Collection stage:
the method of the collection depends on:
– The density of the gas in comparison to air.
– The solubility of the gas in water.
Main methods of collecting gases;
a) Overwater

The gas displaces the water from the gas jar. This method is known as the downward displacement of water.
N.B: the method is used only if the gas is insoluble in water (such as hydrogen and oxygen and if not required to be dry.
(b) Upward delivery or downward displacement of air.
Ammonia gas and dry hydrogen are collected by the above method since they are less dense than air and ammonia is soluble in water.

(c) Downward delivery or upward displacement of air
Carbon dioxide (CO2), sulphur dioxide (SO2), Nitrogen dioxide (NO2), chlorine and hydrogen chloride (HCl) are collected by this method because they are denser than air and are soluble in water.
(d) By liquefaction and freezing

Nitrogen dioxide (NO2) can be prepared in a liquid state by using a cool method.
HYDROGEN
Industrial preparation
By electrolysis of dilute sulphuric acid or acidified water using platinum electrodes. Hydrogen gas is liberated at the cathode and oxygen at the anode
The reaction at the cathode
2H+(aq) + 2e → H2(g)
The reaction at the anode
4OH–(aq) – 4e → 2H2O(l) + O2(g)
Laboratory preparation of hydrogen
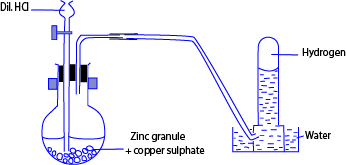
METHOD
- Put some zinc granules in the conical flask. Carefully (do not drop), then fix a cork carrying a thistle funnel, and a delivery tube.
- Arrange the apparatus pour some copper sulphate through the funnel, followed with hydrochloric acid and let it get in contact with the zinc.
- Collect the gas
Test for the gas;
Method: Insert a burning splint into a jar full of hydrogen.
Observation: a pop sound
Properties of hydrogen gas.
- Colourless
- Odourless
- Insoluble in water
- Hydrogen is a reducing agent
- It reduces black oxide to copper to brown metal
CuO(s) + H2(gas) → Cu(s) + H2O(l)
- It reduces yellow lead II oxide to grey metal
- PbO(s) + H2(gas) → Pb(s) + H2O(l)
Uses of hydrogen
- Used as rocket fuel
- In nuclear reactors to generate energy
- To harden vegetable oils
OXYGEN
Oxygen is one of the most important gas found in air. It is used for many purposes e.g. breathing, rusting and burning.
Industrial preparation
By distillation of air of liquid air.
Air is passed through concentrated sodium hydroxide to remove carbon dioxide and then through silicon dioxide to remove the water vapour. The remaining air is rich in nitrogen and oxygen is compressed to about 200 atmospheres and cooled to a pale-blue liquid. The liquid air is fractionally distilled, nitrogen (b.pt. -196oC) boils off first and blue liquid oxygen (b.pt. -183oC) remain.
Laboratory preparation
By decomposition of hydrogen peroxide in presence of manganese (IV) oxide (MnO2) as a catalyst.
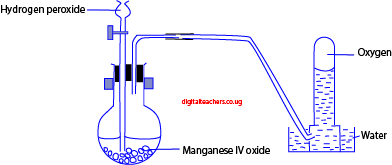
Equation
2H2O2 (aq) → 2H2O (aq) + O2 (g)
Test for the gas given off
Oxygen relights a glowing splint.
CATALYST
Is a substance that speeds up or alters a chemical reaction but it remains unchanged at the end of the reaction.
EXPERIMENT: To investigate the effect of a catalyst on the production of oxygen
You are provided with three test tube labelled A, B, C; to test tube
A put hydrogen peroxide
B put manganese dioxide
C put both manganese dioxide first followed with hydrogen peroxide
Allow the experiment to stand for 10 minutes.
Insert a glowing split in test tube A, B, C respectively
Record your observation and conclusion.
Observation: Oxygen was given off after a long period. The glowing splint kept on glowing.
In B no oxygen was given off.
In C oxygen was given off after a short time. The glowing splint busted into a flame
Conclusion: the manganese dioxide speeds up the production of oxygen from hydrogen peroxide.
Properties of oxygen
- Colourless gas
- Neutral to litmus
- Odourless gas
- Slightly soluble in water
Uses of oxygen
– Breathing
– Burning
– Rusting
ACTION OF OXYGEN WITH METALS & NON – METAL:
Oxygen combines vigorously with many metals and non-metals forming basic and Acidic oxides respectively.
– METALS + OXYGEN → METALLIC OXIDES
Most of the oxides are basic in character (basic oxide react with acids to form salts if they are soluble, they form solutions that Red litmus blue
– NON – METAL + OXYGEN → NON – METALLIC OXIDE
Most of the oxides are acidic in character and turn blue litmus red.
EXPERIMENT: To investigate the action of heat on:
- Metals
- Non-metals
You are provided with metals and non-metals, burn sodium metal in air and dissolve the oxide in water and test the resultant mixture with litmus paper both blue and red. (Do this with sodium for metals and sulphur for non-metals).
Write your observation and conclusion. Continue burning the other metals
1. Sodium (Na)
Burns with a bright yellow flame and form a yellowish solid
2Na(s) + O2(g) → Na2O2(s)
Na2O2(s) + H2O(l) → 2NaOH(aq) + O2(g)
2. Calcium (Ca): Burns with a bright red flame and form a white solid
2Ca(s) + O2 (g) → 2 CaO(s)
CaO (s) + H2O(l) → Ca(OH)2 (aq)
3. Zinc: On burning, it decomposes to a yellow solid when hot and turns white on cooling.
2Zn(s) + O2(g) → 2ZnO(s)
4. Lead: Melts and then forms yellow oxides when hot and yellow when cold
2Pb(s) + O2(g) → 2PbO(s)
5. Magnesium: burns in oxygen with a very bright light and forms light white ash.
2Mg(s) + O2(g) → 2MgO(s)
Mg(s) + H2O(l) → Mg (OH)2 (aq)
6. Copper: burns with a green flame
2Cu(s) + O2(g) → 2CuO (s)
7. Iron filings (or wire): burn with a shower of bright sparks and forms a blue-black solid insoluble in water
3Fe(s) + 2O2(g) → Fe3O4(s)
NON-METALS
Burn sulphur in the air and dissolve the gas in water test the resultant mixture with both red and blue litmus papers.
Write your observations and conclusion
1. Sulphur: Burns with a bright blue flame and forms cloudy fumes with a chocking smell
S(s) + O2 (g) → SO2(g)
SO2(g) + H2O(l) → H2SO3 (aq)
2. Carbon: burns with an orange flame and makes bright sparks
C(s) + O2 (g) → CO2(g)
CO2(g) + H2O(l) → H2CO3 (aq)
TYPES OF OXIDES
Basic oxide:
is a metallic oxide, which reacts with an acid to produce a salt and water only. When dissolved in waters, it forms an alkaline solution
i.e. CaO(s) + 2HCl(aq) → CaCl2(aq) + H2O(l)
(salt) water
CaO(s) + H2O → Ca(OH)2 (Alkaline)
Acidic oxide
These are oxides of nonmetals, they dissolve in water to form acids
SO2(g) + H2O(l) → H2SO3(aq)
Amphoteric oxide:
is a metallic oxide, which can show both basic and acidic properties. (it reacts with both acids and bases to produce water only and a salt e.g.
ZnO, Al2O3, PbO
Neutral oxide:
is an oxide which shows neither basic nor acidic character e.g. H2O, CO
PRODUCTS FORMED WHEN A CANDLE BURNS IN OXYGEN
Candle wax is a mixture of hydrocarbons, which are compounds of hydrogen and carbon
To find the product formed light the candle, place it under the funnel and use a pump to suck the hot gases through lime water(calcium hydroxide solution) to absorb carbon dioxide. Arrange the apparatus as shown below. Allow the experiment to stand for 5 minutes, put out the candle.

Observations
The vapour condenses in the U-tube and lime water turns milky. When a drop of the condensed vapour is added to anhydrous copper sulphate. It turns from white power to blue crystals OR when the drop is added to anhydrous cobalt chloride, it changes from blue to pink.
CxHy + (x+ y/4 )O2 (g) → xCO2 (g) + yH2O(l)
Conclusion
Therefore, when a candle burns in oxygen, water vapour and carbon dioxide are produced. There is an increase in mass equal to the mass of oxygen that has combined with the wax.
Carbon dioxide
By reacting an acid and a carbonate. Usually, hydrochloric acid and calcium carbonate (marble) are used because they are cheap.

Carbon dioxide is collected by downward delivery or upward displacement of air.
Note
- that sulphuric acid is not normally used to react with marble because calcium sulphate is insoluble that it stop the reaction before much hydrogen is produced.
- All carbonates other than those of sodium and potassium decompose to release carbon dioxide. For example
CaCO3 (s) → CaO(s) + CO2 (g)
3. Hydrogen carbonates of potassium and sodium decompose to produce carbon dioxide
2NaHCO3 (s) → Na2CO3(s) + CO2 (g) + H2O(l)
4. All carbonates are insoluble in water apart from those sodium and potassium.
Testing for carbon dioxide
It turns lime water milky due to the formation of insoluble calcium carbonate.
Ca(OH)2 (aq) + CO2(g) → CaCO3(s) + H2O(l)
With excess carbon dioxide, the milky substance dissolves to form a clear solution due to the formation of soluble calcium hydrogen carbonate.
CaCO3(s) + H2O(l) +CO2(g) → Ca(HCO3)2
Physical properties
- It is colourless
- Odourless
- Heavier than air
- Slightly soluble in water to form an acidic solution
CO2(g) + H2O(l) → H2CO3(aq)
Chemical properties
- Reacts with alkalis to form carbonates and in excess, it forms hydrogen carbonates
2NaOH (aq) + CO2 (g) → Na2CO3(aq) + H2O(l)
Then
Na2CO3(aq) + CO2(g) + H2O (l) → 2NaHCO3(s)
NB.
- Sodium hydrogen carbonate is less soluble than sodium carbonate, thus prolonged addition of carbon dioxide in sodium hydroxide finally produces white crystals of sodium hydrogen carbonates.
- Sodium and potassium hydrogen carbonates are the only solid hydrogen carbonate.
- It reacts with magnesium to form white magnesium oxide powder and black carbon
2Mg(s) + CO2(g) → 2MgO(s) + C (s)
4. Through photosynthesis by green plants, it is converted to glucose
6CO2(g) + 6H2O(l) (photosynthesis) → C6H12O6 (glucose) + 6O2(g)
Uses of carbon dioxide
- Used in refrigerator
- In fire extinguishes
- In soft drinks
- For photosynthesis
For revision question and answers download PDF below

very wonderful keep up
Can you make PDFs please.but thanks for the work
Check on “digitalteachers.co.ug”
Your blog is a treasure trove of information. Bags Wallet & Luggage
I couldn’t agree more. Well said! 500 ka redeem code
Plan your budget with accurate details about the MBBS Fees Structure in Bihar.
Find out how MBBS Direct Admission in Telangana can help you.
Enjoy exclusive benefits by applying the Raja Luck Invite Code.